What Is Light Sheet Microscopy?
Light sheet microscopy is an extremely versatile technique with a vast range of implementations that are typically designed with the needs of the sample in mind.
The underlying principle is the same; the sample is illuminated with a sheet of light positioned perpendicular to the imaging axis which only illuminates the plane in focus. However, imaging and mounting geometries can be very different between the techniques.
Here, we explain more about what light sheet microscopy is and present information on the most popular light sheet techniques. We also give advice on choosing cameras to best match the needs of the application.
For a quick overview of what light sheet microscopy is, take a look at our short article: What is Light Sheet Microscopy?

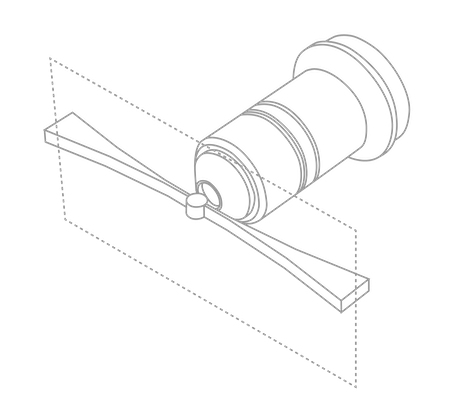
Light Sheet Microscopy
The aim of light sheet microscopy is to image a typically large sample with short time intervals under healthier conditions over longer periods (e.g. days) than conventional light microscopy techniques.
By illuminating one plane of the sample at a time, the sample is exposed to less light, improving sample lifetime and reducing the impact of out-of-focus light.
OpenSPIM
There is a broad range of commercial light sheet systems on the market that offer a solution for almost every application. However, commercial systems can be expensive and may not necessarily fit the exact needs of the sample.
In an effort to make light sheet technology available to everyone, the OpenSPIM platform was created to provide a maximally cost-effective solution that allows anyone to build an entry level system and further tweak it for their specific imaging needs.
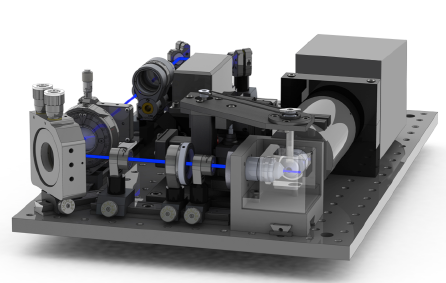
iSPIM and diSPIM
Inverted Selective Plane Illumination Microscopy (iSPIM) and dual-view iSPIM (diSPIM) use a light sheet geometry that introduces the light sheet from both sides of the sample whilst simultaneously acquiring images.
The diSPIM system creates 3D images by collecting volumes from perpendicular directions and computationally combining data from the lateral views to create a volume with isotropic resolution (330 nm) in x, y, and z.
In this way, the poor axial resolution from one view is overcome by information from the other view.
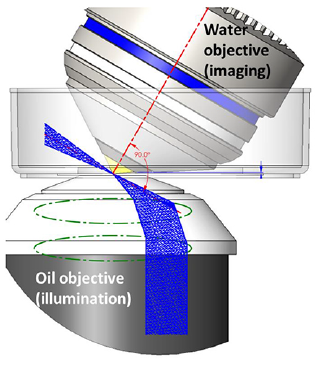
oSPIM and doSPIM
Oblique Single Plane Illumination Microscopy (oSPIM) and dual-view oSPIM (doSPIM) generate the light sheet at an oblique angle using an oil-immersion objective below the sample. A high NA, water dipping, detection objective sits above the sample and is tilted 60 degrees perpendicular to the light sheet which provides an ideal geometry for cell and tissue culture samples.
oSPIM is a platform for high resolution light sheet microscopy combining the low photobleaching and photodamage of LSFM with high magnification, high NA objectives for cellular and subcellular imaging.

Mizar Tilt
Light sheet microscopy can typically only be performed using low magnification/NA objectives due to the long working distance required. The Mizar Tilt overcomes this problem by removing the illumination objective and introducing a tilted light sheet through a photomask and cylindrical lens which can be made to converge at the working distance of high NA objectives.
In this way, high magnification and high NA (60x, 1.49), oil-immersion objectives can be used to image coverslip-based mounted samples. It can be applied to most existing upright or inverted microscope systems and requires no computational reconstruction to view.
Lattice Light Sheet
Lattice light sheet uses Bessel beams to generate ultrathin light sheets from 2D optical lattices to perform light sheet imaging at super-resolution levels. Lattice light sheet allows for 4D imaging of hundreds of samples at sub-second intervals with overall less intensity than a standard light sheet.
The Bessel beam is projected onto a spatial light modulator (SLM), which can change the waveform and introduce a structured pattern. This kind of beam shaping allows for thinner light sheets and higher resolutions.
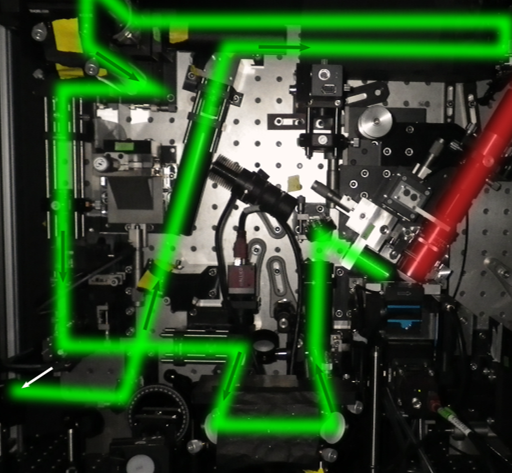
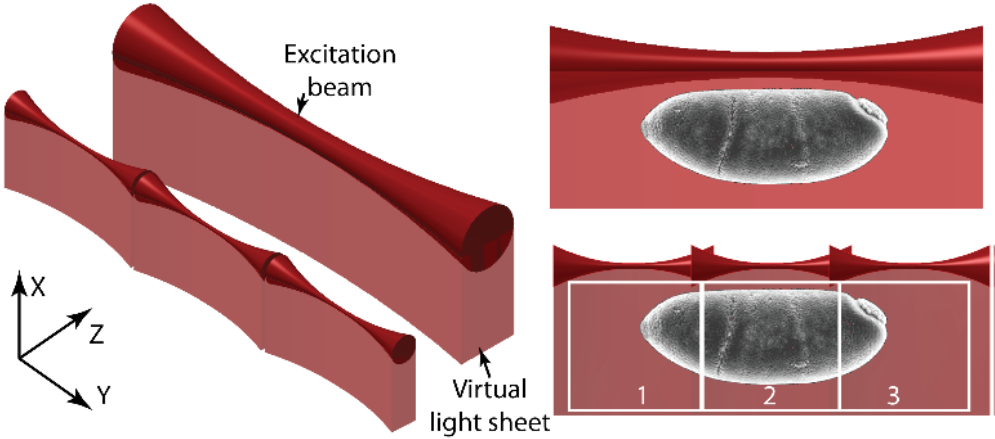
Tiling Light Sheet
Tiling light sheet (or TLS-SPIM) was developed as a solution to the compromise between spatial resolution, optical sectioning capability and field of view (FOV) that takes place with most light sheet techniques. A different approach to light sheet was necessary in order to compromise no longer.
Tiling light sheet involves ’tiling’ multiple smaller light sheets together, creating several very thin light sheets. These smaller light sheets have improved optical sectioning and image resolution, but reduced FOV. By reconstructing the images together, the entire FOV can be shown. This allows light sheet to have good resolution and optical sectioning, even over large FOVs.
Cameras for Light Sheet Microscopy
The large number of different light sheet microscopy techniques makes it difficult to be sure that you’re getting the right camera.
In this technical note, we take a look at typical camera specifications used for light sheet techniques and make recommendations from our product range.

