Dr. Scott Cruikshank
Research Associate Professor of Neuroscience
Brown University, Rhode Island, USA
Background
Dr. Cruikshank and colleagues are interested in information processing in the brain, focusing on circuits linking the brain’s neocortex and thalamus. These circuits are critical to sensation, perception and learning. Using optogenetic probes, fluorescent and bioluminescent proteins and electrical recording, they can activate and or inhibit particular pathways and measure changes in downstream processing.
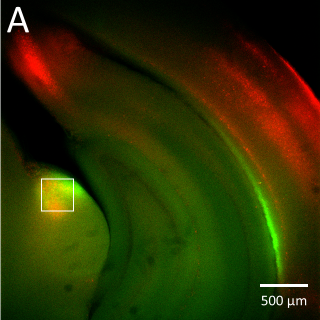
(B) Higher magnification image of the corticothalamic ChR2-EYFP terminal labeling at the approximate position of the boxed region in panel A (imaged with a 40x water immersion objective). The high sensitivity of the Prime BSI allowed identification of the corticothalamic terminal zone using relatively low light intensity, preventing unnecessary activation or bleaching of the opsin and fluorescent protein.
Challenge
The group often image axon terminals that contain channelrhodopsin and other opsins tagged with fluorescent proteins. These terminals are fine structures and are sometimes only weakly fluorescent, causing problems with signal to noise in the resulting images.
Dr. Cruikshank told us, “We have typically used a fast sCMOS camera that can acquire images at 100 Hz (full frame) but the quantum efficiency of that camera is just 60%, and the signal to noise ratio is generally low at fast frame rates.”
Common solutions to weak signals can include illuminating with higher intensities or for a longer duration, but this can excessively activate the channelrhodopsins used to control the neuronal excitability. Uncontrolled activation of the channelrhodopsins can lead to prolonged changes in neuronal behavior, confounding the results of the experiments. On the other hand, lowering the illumination intensities and/or exposure times enough to minimize channelrhodopsin activation can degrade the ability to detect and image labeled nerve terminals.
Using the Prime BSI to image weakly illuminated and sparsely labeled fluorescent neurons, we are able to avoid uncontrolled activation of channelrhodopsin while imaging the signal in axon terminals using short exposure times
Solution
The group is now using the Teledyne Photometrics Prime BSI back-illuminated sCMOS camera.
Dr. Cruikshank told us, “The Prime BSI, with 95% quantum efficiency and 6.5 μm pixels, was a great solution to our issues of light detection and required resolution. We were also very happy to see that the fixed pattern noise was lower than that of our previous sCMOS camera.”
He continued, “Using the Prime BSI to image weakly illuminated and sparsely labeled fluorescent neurons, we are able to avoid uncontrolled activation of channelrhodopsins while imaging the signals in axonal terminals using short exposure times”.
Dr. Cruikshank concluded, “We are happy with the Prime BSI and also with Teledyne Photometrics as a company. The service and support from Teledyne Photometrics has been very good. Their people are helpful, patient, experienced, and seem to have excellent technical knowledge.”

