Dr. Scot Kuo
Microscope Facility Director and Associate Professor
in Biomedical Engineering and Cell Biology
Johns Hopkins University School of Medicine
Background
The Johns Hopkins University School of Medicine Microscope Facility has an expansive selection of research microscopes, including multiple laser scanning confocal microscopes, a multiphoton microscope, an atomic force microscope, a TIRF microscope and multiple spinning disk confocal microscopes. The wide variety of microscopy options allows the university team to work with researchers to develop techniques for specialized research projects.
Challenge
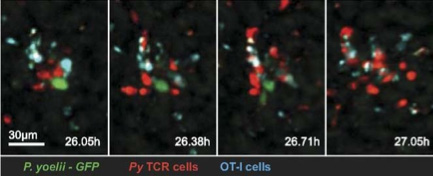
To understand the cellular immune response protecting against malaria infection in its early liver stage, a colleague from the Johns Hopkins Malaria Research Institute approached the team about conducting in vivo imaging of infected mice. Malaria parasites and specifically activated T-cells (CD8+) are both capable of extremely fast motility in liver tissue. Using a non-fluorescent mouse host, their colleagues wanted to monitor the motility of injected parasites and T-cells, each expressing different fluorescent proteins. To address specificity, non-activated control T-cells labeled with a third fluorescent protein are often included in the same mice. In addition to choosing an appropriate microscope system, additional challenges of this experimental system includes the photosensitivity of these labeled cells and the high autofluorescence of liver tissue. Fast motility requires fast imaging, but the phototoxicity limits excitation intensity.
Due to phototoxicity and speed of imaging, the Evolve 512 EMCCD camera is essentially running at single-molecule sensitivities in our experiments. We conducted side-by-side camera comparisons… Only the Evolve was suitable for this research.
Solution
On an Intelligent Imaging Innovations (3i) system based on the Yokogawa spinning disk confocal microscope, the team selected the Photometrics Evolve® 512 EMCCD camera (new series now available) as the best camera solution. Although the spinning disk confocal microscope limits the effects of the tissue’s autofluorescent ‘haze’, the exceptional signal-to-noise of the Evolve camera provided the additional discrimination needed to see labeled cells. Given the 3D motility of T-cells, z-stacks are required, so the system is running continuously to provide the spatiotemporal resolution needed. It cannot run any slower for fear of missing abrupt changes in motility as T-cells ‘discover’ and attack malaria parasites.
To minimize phototoxicity, the 3i system has the excitation lasers electronically slaved to the Evolve 512’s ExposeOut synchronization signal. The lasers are only active when the camera is collecting photons, thus limiting exposure times to the absolute minimum. Even so, the lasers are running at very low power levels to preserve cell viability. Dr. Kuo explains, “Due to phototoxicity and speed of imaging, the Evolve is essentially running at single-molecule sensitivities in our experiments. We conducted side-by-side camera comparisons on the same microscope system, including two different sCMOS cameras and a less deeply cooled EMCCD camera. The results clearly showed that these other cameras were inappropriate. Cells were lost in the noise even though exposures were ~250ms. Only the Due to phototoxicity and speed of imaging, the Evolve is essentially running at single-molecule sensitivities in our experiments. We conducted side-by-side camera comparisons… Only the Evolve was suitable for this research.” The team’s malaria experiments continue and have expanded their research to include other tissues (skin) and other stages of malaria infection (transfer into blood vessels).

Further Information
More about the Microscope Facility at John Hopkins University is available at: http://www.hopkinsmedicine.org/micfac/
More about advanced imaging technologies for cell and subcellular studies is available at: www.jhu.edu/cmml/
