Spinning Disk Confocal Microscopy
Confocal microscopy addresses two significant challenges in biological imaging that conventional fluorescence microscopy cannot overcome. Firstly, biological specimens are 3-dimensional structures so to fully understand them we often need to construct 3-dimensional images. Secondly, many processes biologists would want to study occur inside biological structures, but other cell features can block a clear view.
Confocal microscopy uses pinholes which allow for optical sectioning to take multiple, thin, 2-dimensional slices of a sample to construct a 3-dimensional model from them. Spinning disk confocal microscopy increases the speed of this technique by using multiple pinholes etched into an opaque disk which, when spun, scans the pinholes across the entire image.
For a quick overview of what spinning disk confocal microscopy is, take a look at our short article: What is Spinning Disk Confocal Microscopy?


Introduction to Spinning Disk Confocal Microscopy
Confocal microscopy uses optical sectioning to take multiple, thin, 2-dimensional slices of a sample to construct a 3-dimensional model from them. This process removes the out-of-focus light from other planes.
Compared to other optical sectioning techniques, spinning disk confocal microscopy is high-speed, high-sensitivity and simple to implement. This makes it a very common choice for studying 3-D structure, fast dynamic processes, long-term time-lapse or details inside the cell membrane, all possible with live cells.
Super-Resolution Spinning Disk Confocal
Spinning disk confocal microscopy is a versatile and widely used imaging technique in biology due to its ability to perform fast, 3D imaging of live cells.
Recently, techniques have been created that combine the simplicity and optical sectioning capability of spinning disk confocal with super-resolution imaging to create a spinning disk system capable of a twofold resolution improvement over the diffraction limit.

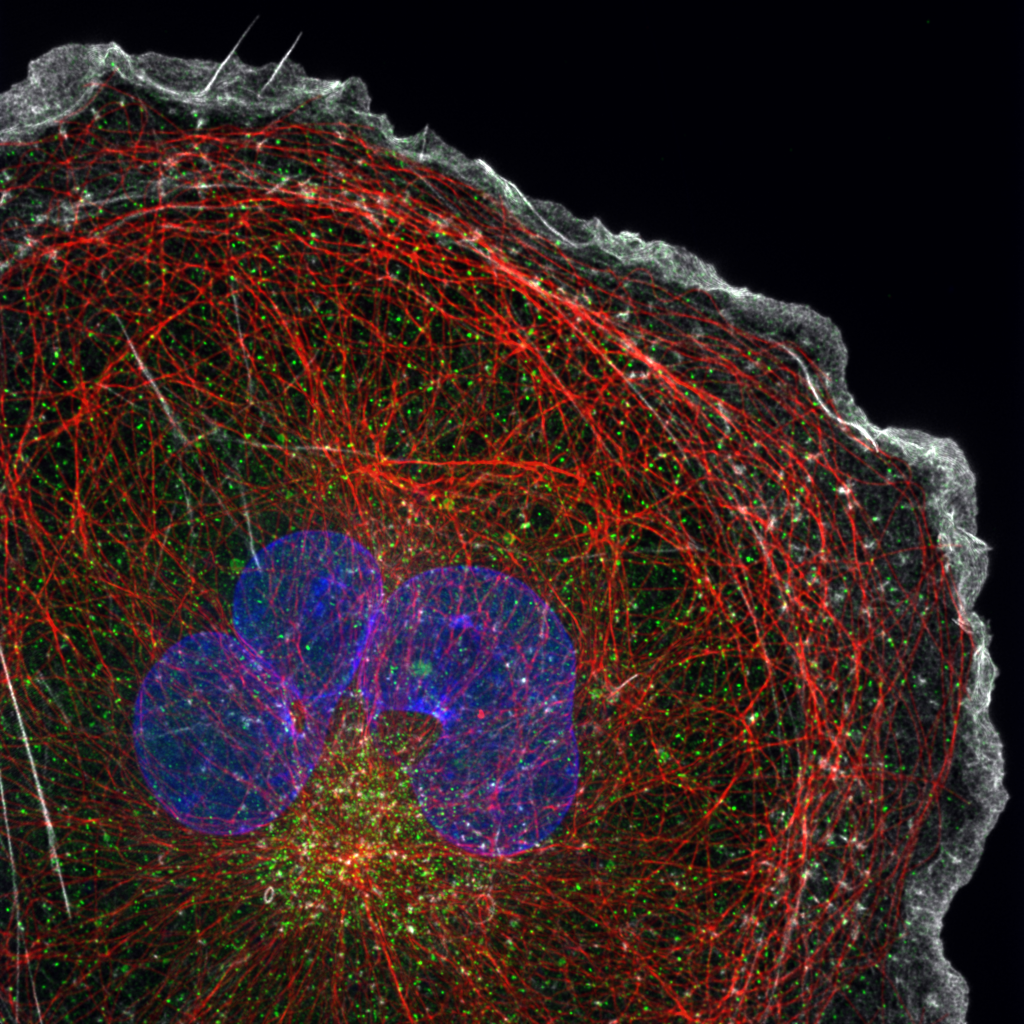
Image Restoration Through Deconvolution
When light from a fluorophore passes through the optics of a microscope, diffraction causes the light to spread. This causes the captured signal to be distorted and take on the characteristic shape known as a Point Spread Function (PSF).
Every photon that is detected by a scientific camera has been spread in this characteristic way – mathematically, this is represented by the convolution of the ‘true’ image and the PSF.
Deconvolution looks to reduce or remove this optical distortion.
Yokogawa Spinning Disk Confocal
The Yokogawa spinning disk unit consists of two coaxially aligned disks, a collector disk and a pinhole disk, with a dichroic mirror positioned between them.
Each disk contains pinholes arranged in a series of nested spirals. The pinholes on the collector disk contain Fresnel microlenses that focus light onto pinholes laterally co-aligned on the pinhole disk where the pinholes are at the focal plane of the microlenses.
When the disks are spun, the pinholes scan across entire image rows in sequence. The holes are positioned so that every part of the image is scanned as the disk is turned.


CrestOptics X-Light Confocal Imager
Conventional spinning disk confocal microscopy uses a dual disk strategy to increase the amount of light reaching the sample by focusing the excitation light through microlenses on the first disk into the pinholes of the second disk. Emission light then passes back through the second disk and onto the camera.
The use of two disks and microlenses is, however, expensive. The X-Light confocal imager is a spinning disk system with a proprietary single disk pinhole design with high light collection efficiency without microlenses to create an affordable spinning disk confocal system.
Swept-Field Confocal Microscopy
Conventional spinning disk confocal microscopy does have drawbacks, such as crosstalk and the acquisition speed which is ultimately limited by the rotational speed of the disks.
Swept-field confocal microscopy overcomes these limitations by using a positionable aperture plate containing a variety of pinhole columns and slit apertures instead of pinholes embedded on a spinning disk. This one-dimensional pinhole/slit array can be swept across the sample by galvanometric and piezo-controlled mirrors to collect confocal information from structures within the plane of focus and reject out-of-focus information at high speed and high resolution with much-reduced crosstalk.

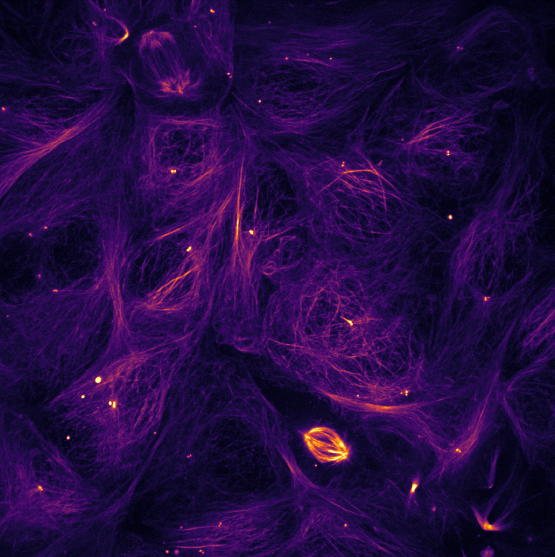
Volocity® Imaging Software
Volocity is an innovative, high performance 3D imaging software product designed specifically for life science research. It allows the user to visualize, explore and analyze multi-channel 3D volumes over time, providing information that is difficult to obtain in any other way.
It’s a complete imaging package that consists of four unique, totally integrated products that provide a suite of tools for 2D, 3D and 4D imaging. These tools are coordinated through Volocity’s unique Library format, which acts as a user-friendly image database for raw and quantified images.
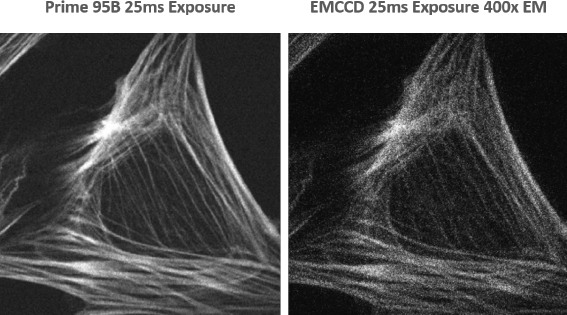
Prime 95B sCMOS for Spinning Disk Confocal Microscopy
Spinning disk confocal microscopy is, essentially, a light rejection technique so one of the main challenges is to collect as many of the emitted photons as possible so light intensity can be reduced to lessen the impact of photobleaching and photodamage on samples.
The high sensitivity of the Prime 95B means that when compared to conventional sCMOS devices, the exposure time could be reduced by up to four times and still give equivalent detection.

The Evolution Of Spinning Disk Confocal
Spinning disk confocal microscopy has advanced greatly as an imaging application since its inception over 50 years ago. At Photometrics we have long supported spinning disk imaging systems with our camera technologies, as both fields experience technological advances.
In this article, see how spinning disk confocal microscopy systems have changed from the past to the present, and how spinning disk could further evolve going into the future.
