Professor John Girkin
Department of Physics and Biophysical Sciences Institute
Durham University, United Kingdom
Background
Prof. Girkin and his multidisciplinary team are interested in applying advanced photonics and optical technology to challenges within the life sciences, in this case imaging live zebrafish. By using selective plane illumination microscopy (SPIM), the beating hearts (1) and developing eyes (2) of live zebrafish can be imaged and analyzed.
Using a homebuilt SPIM imaging system (3) which incorporates a visual heart synchronization method; a variation on SPIM that includes beam scanning (digital SPIM or D-SPIM), and a novel spatial frequency domain imaging system (patent pending), Prof. Girkin and team have a wealth of imaging techniques used to interrogate live samples.
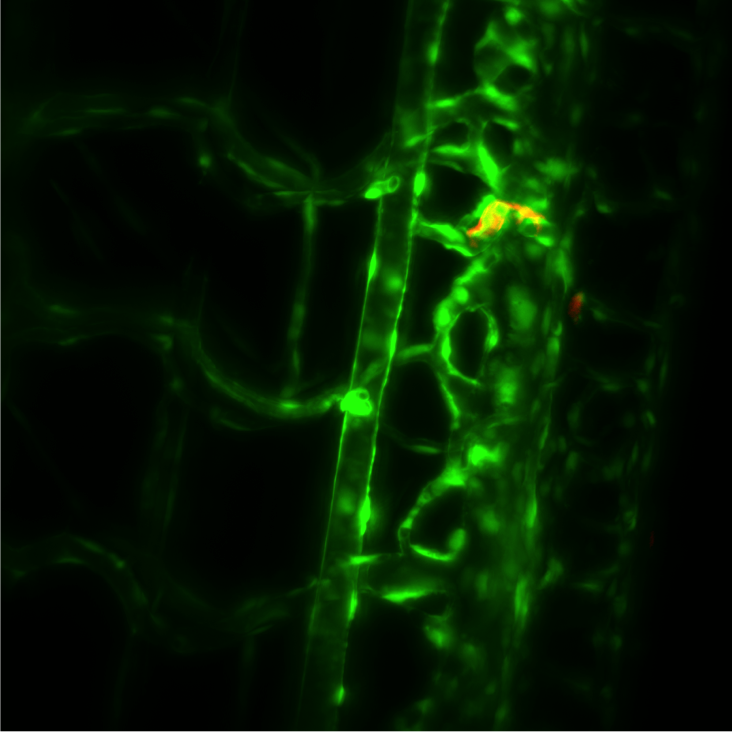
Challenge
In order to visualize the zebrafish heartbeat, Prof. Girkin told us, “a high speed camera, operating in the near infrared, uses transmission images of the beating heart and real-time analysis software to synchronize the light sheet and fluorescence imaging camera to effectively ‘freeze’ the motion of the heart. The fluorescence imaging camera has to have accurate and repeatable shutter operation in combination with high sensitivity, to capture the image at the correct part of the heart’s cycle.”
The D-SPIM system has similar requirements, needing accurate camera control electronics and software to maintain synchronization, and a low noise sensitive camera to take advantage of the method. When imaging the developing zebrafish eye, a good signal is required even in low light conditions over a 24-48 hour study period.
For the novel spatial frequency domain imaging, Prof. Girkin informed us: “This is an excellent method of imaging through tissue, in particular, skin if near-infrared light is used. A highly sensitive camera is therefore required with, in our case, some capability in the near infrared (around 800 nm).”
The Iris 9 is demonstrating high sensitivity to the low light levels present during rapid in vivo imaging and also the timing required to ensure the accurate image synchronization. We have imaged hearts for over an hour without losing timing lock on the imaging, demonstrating the performance of the camera
Solution
The Iris 9 meets all of the imaging requirements for these numerous microscopy and imaging techniques, as confirmed by Prof. Girkin, “We have now replaced our previous camera with the Iris 9 which is demonstrating high sensitivity to the low light levels required for rapid imaging and also the timing required to ensure the synchronization is correct. We have imaged hearts for over an hour without losing timing lock on the imaging, demonstrating the performance of the camera.”
The Iris 9 is working well with the D-SPIM system, and the high sensitivity is utilized when studying the movement of individual cells within the developing zebrafish eye (1), with the Iris 9 “demonstrating significant advantages over our previous camera in this regard.”
References
- Taylor, J. M., Saunter, C. D., Love, G. D., Girkin, J. M., Henderson, D. J., & Chaudhry, B. (2011). Real-time optical gating for three-dimensional beating heart imaging. Journal of Biomedical Optics, 16(11), 116021.
- Young, L. K., Jarrin, M., Saunter, C. D., Quinlan, R. A., & Girkin, J. M. (2018). Non-invasive in vivo quantification of the developing optical properties and graded index of the embryonic eye lens using SPIM. Biomedical Optics Express, 9(5), 3947–3958.
- Girkin, J. M., & Carvalho, M. T. (2018). The light-sheet microscopy revolution. Journal of Optics, 20, 053002.

