Dr. Raghu Padinjat
National Centre for Biological Sciences (NCBS)
Background
The ability to perceive external stimuli and respond accordingly is a fundamental characteristic of biological systems. The stimuli are then converted to signals that can be read by cells via the process of signal transduction, of which there are many different kinds. The G-protein coupled receptor (GPCR) pathway is one such kind of pathway, one particular class of which is driven by the cleavage of lipid molecules known as phosphoinositides, specifically, phosphatidylinositol-(4,5)-bisphosphate [PI(4,5)P2]. This action further propagates the signal and results in a physiological response. The maintenance of PI(4,5)P2 levels is vital for a sustained response to a continuous signal, requiring the recycling of PI(4,5)P2 to be tightly regulated. PI(4,5)P2 resynthesis is a multi-step pathway, distributed over different membranes and involving several lipid intermediates and lipid-modifying proteins. The visual transduction of the fruit fly, Drosophila melanogaster has proven to be an ideal model system in which to study the regulation of GPCR signaling.
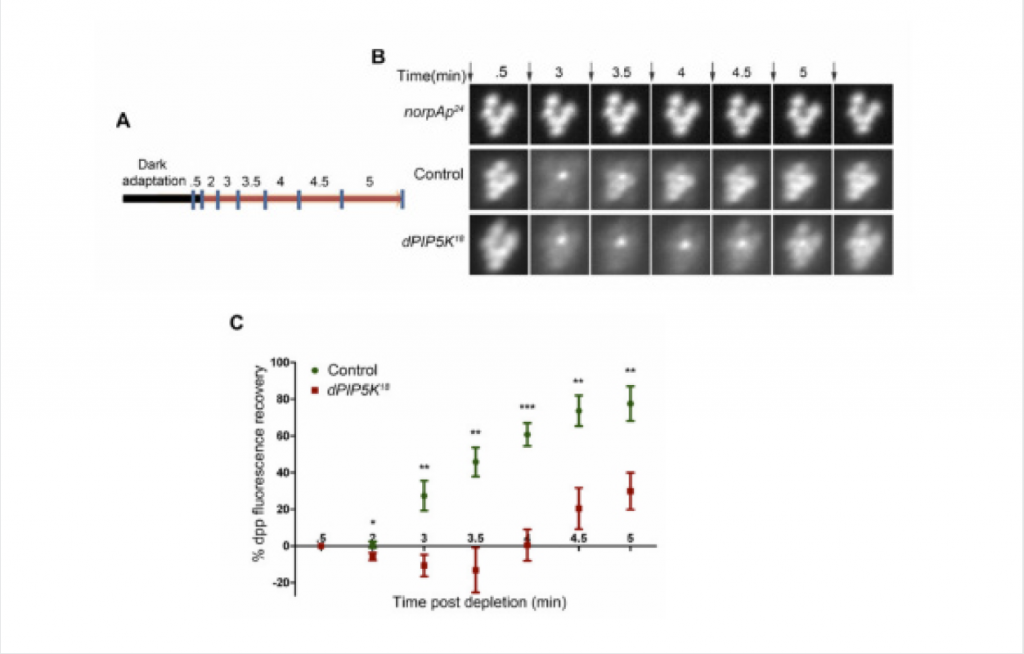
Figure B: Fluorescent deep pseudopupil (DPP) imaging to study PI(4,5)P2 dynamics using flies expressing the PI(4,5)P2 biosensor. The time scale of the imaging is indicated on the top of each panel. Arrows indicate the timing of a 90 ms flash of blue light used for imaging the dpp. Images were acquired from control, dPIP5K18 and norpAP24. The genotypes used for the image acquisition are labeled at the left of the image panel. norpAP24, which is a protein null mutant of PLCβ, is used to show the dependence of DPP dynamics on PLCβ activity.
Figure C: Quantitative representation of PI(4,5)P2 dynamics. X-axis represents time in minutes between the depleting flash of blue light and the next image acquired. During this period eyes were illuminated in red light. Y-axis represents the level of fluorescence represented as a % of the value in the initial image. Error bars represents mean +/− S.D from five flies. p values were calculated using an unpaired t-test. The stars represent level of significance (***p< 0.001; **p< 0.01; *p< 0.05)
Challenge
In order to actually determine the specifics of PI(4,5)P2 turnover during signaling, the movement of the lipid must be monitored. Since fluorescently labelling the lipids in vivo is not a feasible prospect, only alternative methods that enable the ability to follow movement of the lipids involved in GPCR signaling can be used.
Live imaging in whole flies is achieved by expressing fluorescently-tagged, lipid-binding probes in the fly eye, which can be used as a readout of the kinetics of the lipid itself. Drosophila eyes contain various optically active elements, often contributing a significant amount of background noise to the preparation.
Researchers have been seeking imaging-based methods to interrogate cellular lipid behavior, with minimal success. The Evolve® 512 EMCCD camera (new series now available) was selected to support the challenging imaging requirements of this research because it offers a singular advantage — the noise is almost completely eliminated. The camera gives the team the ability to reliably track and analyze the movement of the fluorescent probe alone.
The Evolve’s high sensitivity and dynamic range were integral to the acquisition of high-quality images for analysis.
Solution
Considering the challenges, lipid-binding protein domains can be harnessed as probes, using their movement as a proxy for kinetics of the lipid itself. Invertebrate eyes, being compound eyes, exhibit a phenomenon known as the deep pseudopupil (DPP). In this scenario, the images of several ommatidia (individual units of the eye) are superimposed to form one large, virtual image of a single ommatidium formed on a plane below the surface of the eye. The DPP can be observed using a microscope and fluorescent probes expressed in the photoreceptors can be clearly seen using appropriate fluorescence optics. The PH domain of PLCδ, fused to GFP, has long been used a reliable probe for PI(4,5)P2 and, when expressed in fly photoreceptors, forms a fluorescent DPP. The team’s assay focuses on determining the kinetics of PI(4,5)P2 in different genetic backgrounds, where protein components of the signaling pathway have been perturbed. A live fly prep is used for DPP imaging, where a flash of blue light (ƛmax488nm) is used to both stimulate phototransduction and excite GFP. The blue light used to excite GFP is also the stimulus to rapidly convert the majority of rhodopsin to metarhodopsin thus activating the phototransduction cascade and triggering depletion of PI(4,5)P2. Between the blue light stimulations, photoreceptors are exposed to long wavelength (red – ƛmax660nm) light for incremental time periods, which reconverts metarhodopsin to rhodopsin. The recovery in DPP fluorescence intensity with time indicates translocation of the probe from cytoplasm to cell membrane upon PI(4,5)P2 re-synthesis, giving insight into the timescale of the process.
When asked how the Evolve 512 performed for image acquisition in these experiments, Dr. Raghu Padinjat, principal investigator, shared, “The Evolve’s high sensitivity and dynamic range were integral to the acquisition of high-quality images for analysis.” He adds, “The camera’s high signal-to-noise ratio and EM gain feature allowed the acquisition of the extremely low intensity images required to carry out studies of membrane function using fluorescently tagged lipid-binding proteins.”
In Drosophila, two enzymes exist that synthesize PI(4,5)P2 – dPIP5K and SKTL – from its major precursor PI4P. In order to determine which enzyme is responsible for PI(4,5)P2 synthesis during phototransduction, individual mutants for both proteins were assayed for defects in photoresponses. The DPP imaging assay was successfully used to monitor PI(4,5)P2 kinetics in both wild-type (WT) flies and in flies mutant for one of the enzymes (dPIP5K) that synthesizes PI(4,5)P2 in photoreceptors. While WT flies show a characteristic fluorescence recovery curve for the PLCδPH-GFP probe, mutants for dPIP5K show delayed recovery kinetics when compared to that of WT flies. Mutants for SKTL show no notable defects in phototransduction, pointing to dPIP5K as the enzyme that synthesises PI(4,5)P2 required for the photoresponse.
Team members in the lab hope to incorporate dual-color imaging in the near future. This will allow them to monitor more than one phosphoinositide species at any given time, enabling them to better understand the regulatory mechanisms involved in lipid homeostasis during GPCR signaling. For this, they believe the DV2 two-channel simultaneous imaging system from Photometrics would be an ideal addition to their imaging apparatus.

