Introduction
The brain is one of the most complex objects in the known universe, both in terms of structure and function. This makes imaging the brain an incredibly challenging task, with researchers looking at the movement of signalling ions (see app note on Calcium Imaging), structure of cells and the electrical activity of neurons (see app note on Electrophysiology).
Intrinsic signal optical imaging (ISOI) is a technique used to map dynamics in single cells, brain slices and even – and most importantly – entire mammalian brains. It is label-free, minimally invasive and gives the researcher access to the basic physiology of a functioning brain. All of these benefits come without having to compromise on spatial and temporal resolution which is otherwise only provided by regular imaging techniques that rely on labels and markers. As the use of the latter techniques is undesired in humans, ISOI can be used to confirm observations made with optical imaging techniques in other mammals.
Brain Mapping Techniques
Observing how brain cells function across an area is often referred to as functional brain mapping (FBM) (sometimes electrical stimulation mapping or ESM), and is important not only in neuroscience research, but also for neurosurgery and other medical techniques applied to the brain. This has led to several techniques being developed in order to map out the brain, one of which is the titular ISOI, but several other techniques:
- Direct electrical stimulation (or cortical stimulation mapping): involves physically placing electrodes onto the exposed brain in specific areas to test the function of those areas, stimulating them with electrical signals. Excellent spatial accuracy and temporal resolution, but extremely invasive and can lead to epilepsy.
- Electroencephalography (EEG): involves recording electrical activity in the brain via a grid of electrodes worn on the head, more akin to putting a hat on than exposing the brain. EEG is far less invasive than direct stimulation, and is quick, simple, cheap and widely available technique. However, the signal is far lower and cannot predict where signals are located very accurately.
- Functional magnetic resonance imaging (fMRI): Measures brain activity by looking at the movement of blood through the brain, as active neurons receive more oxygenated blood. fMRI has good spatial but poor temporal resolution, and also requires use of an MRI machine and technical expertise.
For more information on brain mapping techniques, refer to Sagar et al. (2018).
Intrinsic Signal Optical Imaging
ISOI is similar to fMRI in that it relies upon measuring areas of oxygenated vs deoxygenated blood to construe activity, but rather than using an MRI machine it uses light and a scientific camera. ISOI takes advantage of the spectral properties of haemoglobin, which has different absorption properties when oxygenated or deoxygenated.
The basic principle of ISOI is that when brain tissue is directly illuminated, the active areas reflect less light than non‑active areas. The more active a brain area the less light reflected, making highly active areas appear darker. There are three main reasons why neuronal tissues change their optical properties when active: active neurons scatter more light (due to swelling and ion movements), natural fluorophores within the cells react (including haemoglobin) and the changes in blood volume/oxidation.
However, in order to best image the brain tissue it is necessary to directly expose the brain, which makes the process invasive. Parts of the skull need to be removed (or the bone can theoretically be thinned down) in order to find a good location for ISOI signals, and a window can be permanently implanted once localisation is complete. Having this window directly to the brain does affect the neighbouring brain areas, meaning that the data collected via ISOI may not be as physiologically relevant as desired. This is outlined in Fig.1.
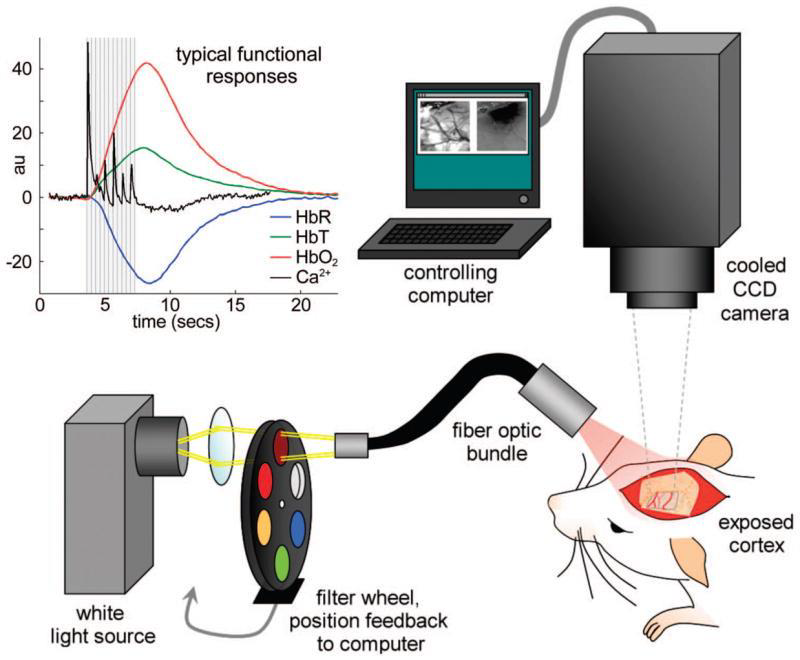
Imaging the intrinsic signal is performed by illuminating the brain with wavelengths between 500 and 650 nm. Repeated measurements are required to obtain a reliable signal detection and localization so a window can be permanently implanted. The window could correct the intrinsic curvature of the cortex but applying pressure on the underlying tissue can result in unwanted artefacts.
The most important reporter used is haemoglobin which has different absorption properties when it is oxygenated or deoxygenated. The general signal for blood flow is imaged by exciting at 540 nm whereas the deoxygenated excitation is imaged at 600 nm. As the extinction coefficient of deoxygenated blood is higher at this wavelength compared to oxygenated, less light will be reflected and reach the camera. In this way, areas of the brain with higher blood flow can be localized. ISO imaging of oxyheamoglobin and deoxyheamoglobin can be seen in Fig.2.
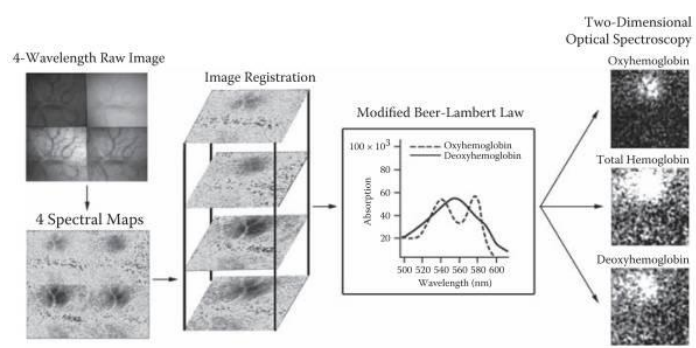
The results are based on calculating the difference between an unstimulated baseline and various time points recorded post stimulation (∆R/R). As signals can be very marginal, the real signal will become obvious only after multiple repetitions of the experiment and averaging across the same time points. Several trials with a repetition of the stimulus paradigm have to be averaged.
Structural changes of the tissue can have a large influence on the reflective properties and hence the outcome of the measurement. During stimulation of the animal, brain areas of interest will enlarge their volume also caused by fusion of secretory vesicles with the pre-synaptic membrane (exocytosis). The excess membrane will be retrieved sooner or later by endocytosis and the tissue volume will normalize again. But for this reason, it is necessary to collect multiple images and use averaging to determine whether the reflective properties are truly indicative of changes in blood characteristics.
To perform ISOI, a mechanically stable setup is required as well as a light source with stable output characteristics. Previously, tungsten-based light sources with nearly no fluctuations in intensity were used but they could result in a reduced signal-to-noise ratio as the variability of the signal could be partially attributed to those fluctuations. Today, the better options are state-of-the-art LED illumination devices which offer high flexibility in terms of wavelength and higher stability over the time course of an experiment.
The biggest problem is to distinguish stimulus-dependent changes in activity from basic activity. Furthermore, although this technique gives unprecedented access in time and space to a brains activity, it’ll only enable the researcher to visualize primary cortices. The imaging depth is dependent on the penetration depth of the used light and typically not more than 500 μm. Areas of interest which are below the cortical surface are out of reach for this technique.
Applications Of ISOI
Despite the need for permanent access to the surface of the brain, ISOI has been used successfully in animal research offering high spatial (~100 µm) and temporal (100 ms) resolution to detect blood flow and oxygen consumption. This has led to important breakthroughs in the organization, physiology, and pathophysiology of the brain. It is hoped that this could lead to the potential clinical use of ISOI in humans.

ISOI has been used successfully to study the principles underlying organization and functional architecture of the brain in several species (including humans), such as cortical development and sensory information processing in vivo. The brain can be imaged as standard or when doing an action, such as moving a limb, obtaining high resolution functional maps from a relatively large area. A number of maps in response to a set of stimuli can be obtained from the same cortical area, which can be imaged repeatedly over a period of weeks or even months. Optical imaging is a technique that combines good spatial resolution, coverage and speed for functional mapping of the mammalian cortex.
Cameras For ISOI
ISOI relies on the detection of signal changes which can occur on the scale of <1% of the signal intensity. Therefore, a high signal to noise ratio to ensure signal detection and a large full-well capacity to detect small signal changes are some of the most desired characteristics of the camera.
Most commonly, CCD cameras are used for ISOI. The high quantum efficiency and broadband sensitivity provide a strong imaging performance. However, CCD cameras tend to have high read noise characteristics which necessitate longer exposure times to have a high enough signal to noise ratio to reliably detect the small changes in signal. Long exposure times are a problem when imaging the live brain because brain movement results in blurred images. The small full-well capacity of CCD cameras can also be an issue for some ISOI applications. For these reasons, some scientific CMOS (sCMOS) cameras are also used.
sCMOS cameras typically have equivalent or better quantum efficiency (up to 95%) than modern CCD cameras with far lower read noise characteristics, giving a much-improved signal to noise ratio. The full-well capacity is also equivalent or better and because of the lower noise characteristics the dynamic range of sCMOS cameras is therefore higher.
Most modern sCMOS cameras also boast larger fields of view (up to 25 mm diagonal) and higher speeds (up to 100 fps, can be made faster by choosing smaller regions). These are typically less important features for ISOI but may be useful for some implementations. For most ISOI applications, CCD cameras are ideal but for those applications where higher sensitivity and dynamic range are required, sCMOS cameras may be the better choice.
Summary
ISOI is capable of fast, contract-free data acquisition using a standard scientific camera with high spatial resolution. It is used in research as a near to none invasive technique and also for diagnostic purposes. Most functional imaging techniques, such as fMRI, have very coarse spatial resolution whereas ISOI is very fine. Furthermore, electrophysiological methods such as single-cell recordings may provide the researcher with excellent knowledge about the individual signal but don’t show the functional context which ISOI does.
Download As PDF
References
Morone KA, Neimat JS, Roe AW and Friedmanc RM. (2017) Review of functional and clinical relevance of intrinsic signal optical imaging in human brain mapping. Neurophotonics 4(3), 031220
Hillman EMC. (2007) Optical brain imaging in vivo: techniques and applications from animal to man. J Biomed Opt. 12(5): 051402. doi: 10.1117/1.2789693
Brennan KC and Toga AW (2009) Intraoperative Optical Imaging. Chapter 13 in In Vivo Optical Imaging of Brain Function, Second Edition. Frontiers in Neuroscience, ISBN 142007685X, 9781420076851, CRC Press
Sobottka, S. B. Meyer, T., Kirsch, M., Koch, E., Steinmeier, R., Morgenstern, U. & Schackert, G. (2013) Evaluation of the clinical practicability of intraoperative optical imaging comparing three different camera setups. Biomed Tech (Berl). Jun;58(3):237-48. doi: 10.1515/bmt-2012-0073.
