Introduction
Electrophysiology is the study of electrical properties of biological cells and tissues, and can be broadly split into neural (nerves throughout the body, especially the spine and brain, also the retina) and cardiac (to do with the heart) electrophysiology.
Electrophysiology is a vital tool in neuroscience, where the right tools can interrogate the activity of neurons and/or astrocytes as they communicate using electrical signals. As well as sensitive electrodes that can to record voltage movements through a cell, sensitive imaging equipment is also needed for high-speed applications such as calcium imaging, or high-resolution applications such as high-content imaging of fluorescently-tagged neurons in brain tissue. Read on for an introduction to electrophysiology and the use of imaging hardware in these applications!
What Is Electrophysiology?
All animal cells are surrounded by a cell membrane, composed of a double layer of lipids with embedded proteins embedded, as seen in Fig.1. While standard electricity (such as the electricity that powers your home) mainly involves the movement of electrons, electrophysiology involves the movement of ions (atoms that have a charge). In the body, some of the most common ions are sodium (Na+), potassium (K+), calcium (Ca2+) and chlorine (Cl–). The area inside cells (cytosol) and outside cells are filled with millions of ions, and the cell membrane acts to control the movement of ions in and out of the cell. This movement of ions across the membrane can be measured and forms the majority of electrophysiological experiments.
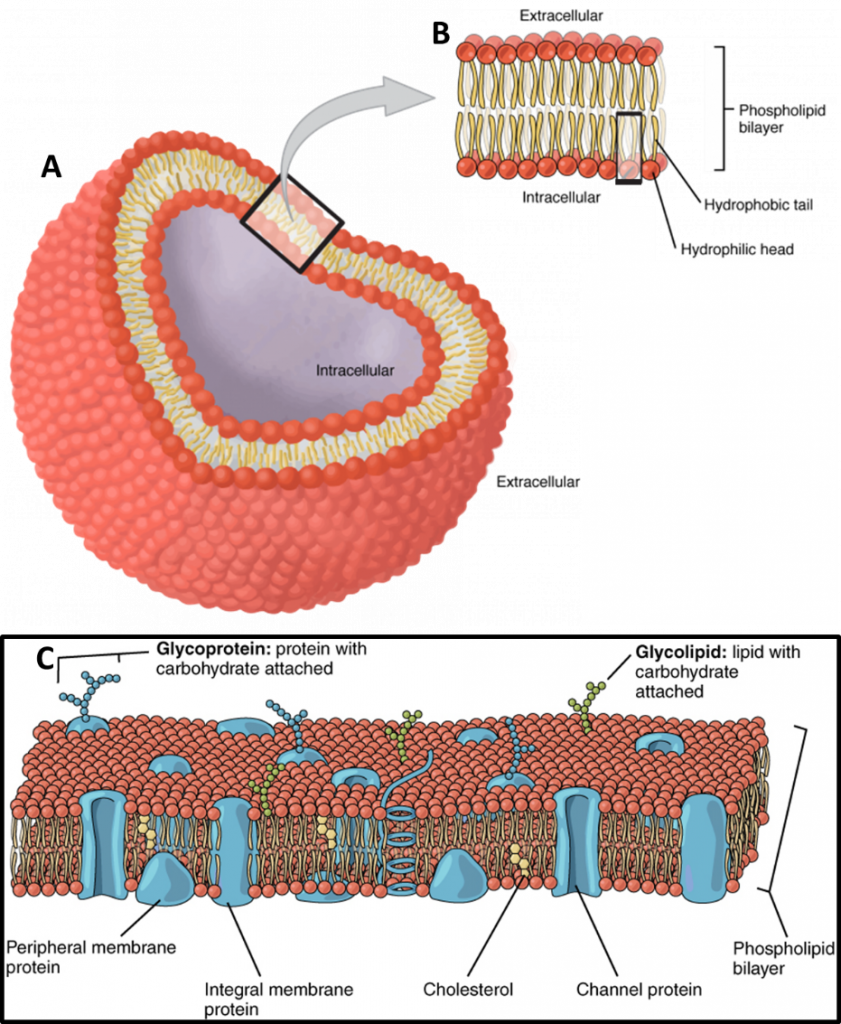
Channel proteins (also known as transmembrane proteins) act as ion transporters/pumps and move ions across the membrane in either direction. Depending on the ion, there are usually more inside the cell than outside, or vice versa, which creates a concentration gradient. The ion channels work to maintain these concentration gradients, for example making sure that there are more K+ ions intracellular, or more Na+ and Cl– ions extracellular. This concept of concentration gradients is outlined in Fig.2.
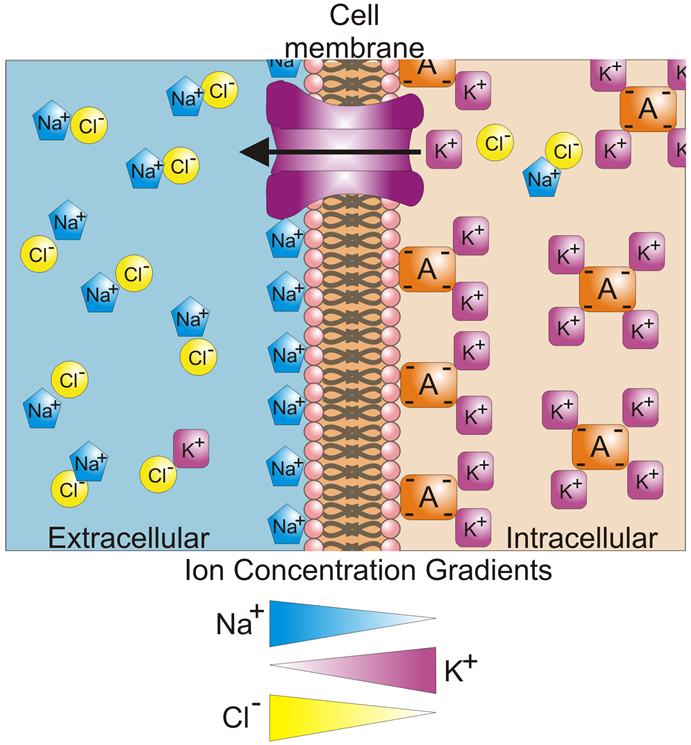
The constant presence of these concentration gradients leads to a difference in the potential (with potential being another word for voltage) between the interior and exterior of a cell. This is called the membrane potential and is typically between ‑40 mV and -80 mV. For most cells, the membrane potential is stable at these typical values, which is also termed the resting potential.
While this resting potential can be measured in electrophysiology, the type of cell that is usually studied is electrically excitable cells, which can change their membrane potential and communicate using electrical activity. Excitable cells are typically neurons or muscle cells, particularly cardiac muscle. By measuring the activity of cells at various membrane potentials, researchers can gain valuable information about ion transport mechanisms, how cells communicate, muscle contraction, and diseases that affect these normal processes. In general, electrophysiology can be divided into two types, intracellular electrophysiology, and extracellular electrophysiology.
Intracellular Electrophysiology
Intracellular electrophysiology involves obtaining electrical information from the interior of a cell. As the cell membrane is an effective barrier to ions, the main way to determine the intracellular activity is to physically pierce through the cell membrane or to isolate a small patch of the membrane with extremely small glass tubes called micropipettes. A micropipette contains electrodes that come into contact with the cell membrane and/or the cytosol within the cell, with one micropipette used per cell. This involves very careful manipulation and technical expertise, meaning that taking intracellular readings is very challenging and often limited to a single cell at a time, but results in very precise information. The gold standard technique is patch clamping, which can be specified for measuring voltage and/or current.
Patch Clamping
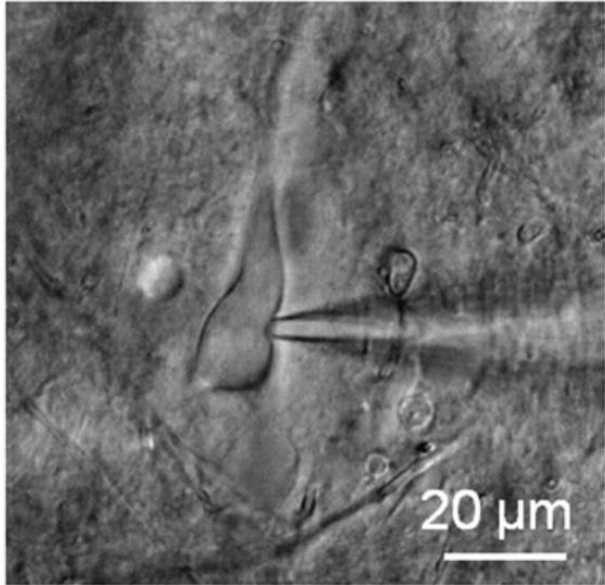
Patch clamping has been in use for over 40 years, with the developers receiving the Nobel Prize in Physiology/Medicine in 1991. In patch clamping, a micropipette is brought into contact with the cell membrane of a single cell, forming an electrical circuit, with an image of patch clamping seen in Fig.3. The micropipette is filled with electrically conductive liquid, and the micropipette tip encloses a small area or ‘patch’ of the cell membrane (approx. 1 µm diameter, which will contain at least one ion channel) and the membrane potential of this area can be recorded. Patch clamping isolates this membrane patch and allows for analysis of cell membranes, and how it responds to changes and how it differs between cell types.
The membrane patch can also be pulled off the cell and within the micropipette for further study. The micropipette can even use suction to break open the membrane while still attached, so that the liquid in the micropipette can mix with the cytosol within the cell, allowing for more data to be recorded. These patch clamp methodologies are outlined in Fig.4.
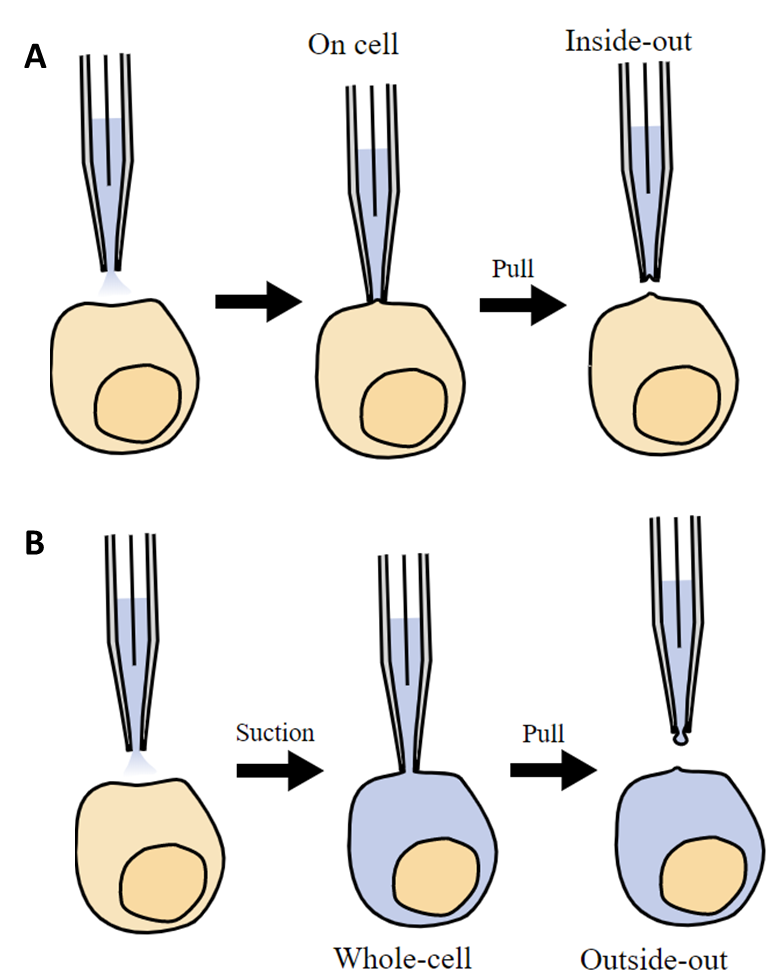
Sharp Microelectrode
As the name suggests, sharp microelectrode involves piercing the cell membrane with a sharp (and small) micropipette, so that the electrode can directly come into contact with the interior of the cell without affecting the ions in the cytosol. The micropipette opening is far smaller so there is no mixing with the cytosol (as seen in patch clamping), and the tip can be filled with different dyes to mark the cells that recordings are being taken from. However, as the cell is being physically speared with a tiny glass spike, permanent damage is done and recordings cannot be taken long term.
Voltage Clamping
Voltage clamping involves using one intracellular recording electrode and another electrode that delivers voltage. While one electrode records the membrane potential, the other electrode can add voltage to the cell, which stimulates the cell membrane and changes the membrane potential. This ‘clamps’ the membrane potential at the desired voltage level, where it is maintained while recordings are taken. Voltage clamping allows researchers to observe cell behavior at different voltages, also using visual recordings to see if the structures inside the cell are still functional.
Current Clamping
Similar to voltage clamping, but instead of seeking to maintain the membrane potential at a set value, current clamping uses a single micropipette to apply a desired current and record how the membrane potential changes in response. By administering repetitive current pulses, the membrane resistance and other biophysical readings can be calculated.
Extracellular Electrophysiology
Rather than obtain recordings from within a cell, it is also possible to take electrophysiological recordings from the exterior of a cell. In general, while intracellular recordings are highly accurate with a great temporal resolution, they are limited by the fact that they can only record from one cell at a time. Extracellular recordings are less accurate as the cell membrane is between the electrode and the signal, but these recordings can be taken quickly, easily and from hundreds of cells simultaneously. This makes extracellular recording more suited when the application requires information from a whole population of cells or tissue
While extracellular recording can record from single cells (termed single-unit recording), it is at its best with multi-unit or field recordings. One of the best examples of multi-unit extracellular recording is the multi-electrode array (MEA). MEAs are devices that contain anywhere from tens to thousands of microelectrodes, which act as an interface between cells and circuitry. Typically, cells are grown on the MEA (instead of the usual glass surface) and their physical contact with the electrodes means that extracellular recordings can be taken from anywhere in the cell population. Cells that are spontaneously active, such as neurons, can be recorded non-invasively over the long-term, across a whole population. Fig.5 shows a population of neurons grown on an MEA.

Imaging In Electrophysiology
While electrophysiology focuses on taking electrical recordings from cells, imaging plays an important role in gathering data. Imaging can be used to reinforce electrophysiology, to prove that targets have generated the desired response. Imaging is extensively used with patch clamping to ensure the correct cell is being patched, and high-speed cameras can detect fluctuations in certain ions in order to image activity as it occurs. Several microscopy techniques and methods are used to locate cells of interest and image the cellular functions being generated. Each method is specific to the type of ion or channel being studied and must be carefully reviewed before experimentation.
Fluorescence Microscopy In Electrophysiology
Fluorescent probes and dyes are often used to image ion channels, as well as visualize specific areas of the cell, as seen in Fig.6.
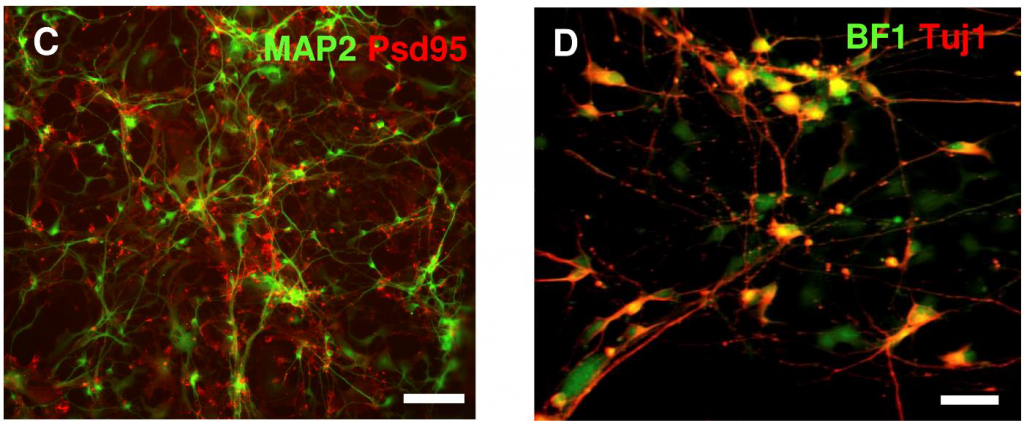
Some examples of fluorescence imaging in electrophysiology include calcium imaging, retinal studies, and optogenetics, as described below.
Calcium Imaging
As electrophysiology involves studying the flow of ions and the resulting effect on the membrane potential of the cell, imaging the ions themselves provides vital information. Calcium imaging involves just that, the imaging of calcium ions (Ca2+) and their role in electrophysiology. This involves the use of calcium indicators, which are fluorescent molecules that respond to the binding of Ca2+ by producing fluorescence, which can be detected. Calcium indicators can either be chemicals that are added to the cell system, or cells can be genetically modified to produce their own (genetically encoded calcium indicators, or GECI).
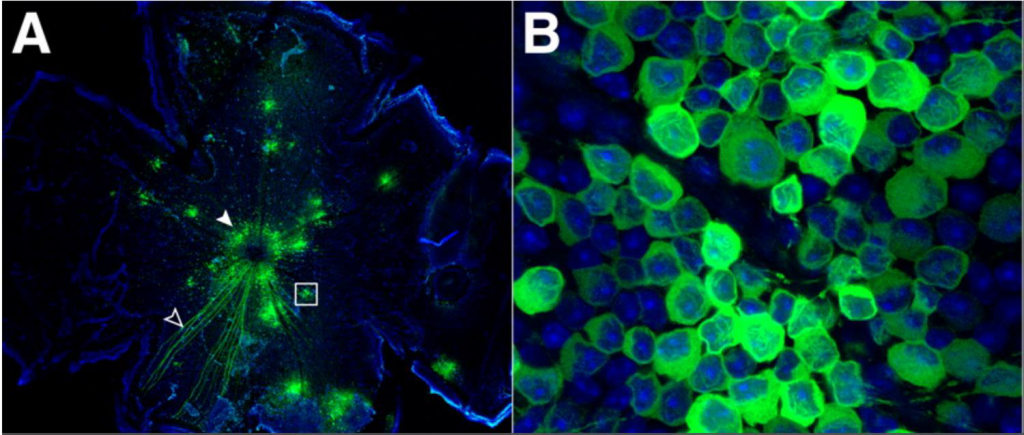
It is vital to choose the right calcium indicator and method of application for the experiment, in order to minimize cell toxicity and maximize the fluorescent effect. With a good signal, the intensity can be used to quantify the amount of Ca2+, enhancing the study. Calcium imaging can be enhanced with the use of a sensitive and high-speed scientific camera, in order to pick up on the signal. An example of calcium imaging can be seen in Fig.7.
Retinal Studies
The retina is widely studied in electrophysiology due to its self-contained sensory circuitry and its usage as a model circuit system. This type of study is termed electroretinography and involves the measurement of the retina’s electrical response to light of varying wavelengths and. Rods, cones, and other electrically active structures in the eye cause measurable changes in the field. These cells are highly structured and benefit from being imaged with fluorescent markers.

Optogenetics
Optogenetics is a technique that involves using light to control cells in living tissues (typically neurons), where those cells have been genetically modified to express light-sensitive ion channels. Optogenetics is a relatively new research technique that has gained a lot of popularity in electrophysiology communities due to its ability to target specific cells within a population.
The use of photoactivatable proteins to generate changes in ion channels has resulted in some fascinating findings for the field of neural microbiology and the study of disease. Often, the activation of the proteins causes a change in the membrane potential. The Deisseroth group used the light‑sensitive protein Channelrhodopsin-2 in the presence of blue light and triggered a brief, but reliable spike in the polarization of the recorded membrane potential of mammalian cells (Boyden et al. 2005). In a separate study, Li and colleagues demonstrated that reliable control of neural functions could also be achieved by using two light-sensitive proteins (Li et al. 2005).
Cameras For Electrophysiology
As electrophysiology is such a varied field, it is vital to utilize scientific cameras that are able to deliver and be flexible across a wide range of experiments. If using cameras for documentation or to support other techniques (such as patch clamping), it is important for the camera to be high resolution at relatively low magnification, requiring a small pixel size. This also applies to high-content imaging of fluorescently-tagged brain tissues.
For specific applications such as calcium imaging, the most important aspect is speed, as these signals move very fast and require high framerates in order to capture and quantify these signals.
Summary
Studying how the electrical environment of the cell can affect the flow of ions and thus, the functionality of cell, is studied widely for its applicability to disease prevention and remediation. Knowledge of electrophysiological techniques as well as the common complications can lead to higher quality data generation. Combining electrophysiology with other techniques such as optogenetics has contributed even further to the field of neuroscience and maintains the status of electrophysiology as a vital technique to our understanding of human biology.
Download As PDF
References
1. Andrásfalvy, B. K., Galiñanes, G. L., Huber, D., Barbic, M., Macklin, J. J., Susumu, K., Delehanty, J. B., Huston, A. L., Makara, J. K. & Medintz, I. L. (2014) Quantum dot–based multiphoton fluorescent pipettes for targeted neuronal electrophysiology. Nature Methods. 11, 1237–1241. doi: 10.1038/nmeth.3146
2. Annecchino, L. A., Morris, A. R., Copeland, C. S., Agabi, O. E., Chadderton, P. & Schultz, S. R. (2017) Robotic Automation of In Vivo Two-Photon Targeted Whole-Cell Patch-Clamp Electrophysiology. Neuron. 95, (5), pp1048–1055. doi: 10.1016/j.neuron.2017.08.018.
3. Borghuis, B. G., Tian, L., Xu, Y., Nikonov, S. S., Vardi, N., Zemelman, B. V. & Looger, L. L. (2011) Imaging Light Responses of Targeted Neuron Populations in the Rodent Retina. J Neurosci. Feb 23; 31(8): 2855–2867. doi: 10.1523/JNEUROSCI.6064-10.2011
4. Boyden, E. S., Zhang, F., Bamberg, E., Nagel, G. & Deisseroth, K. (2005) Millisecond-timescale, genetically targeted optical control of neural activity. Nat. Neurosci. 8, (9), pp.1263–1268. DOI: 10.1038/nn1525
5. Deisseroth, K. (2011) Optogenetics. Nature Methods. 8, 26–29. doi:10.1038/nmeth.f.324
6. Ding, S., Mattam, S. G. & Zhou F. M. (2011) Kv3-like potassium channels are required for sustained high-frequency firing in basal ganglia output neurons. J Neurophysiol. Feb;105(2):554-70. doi: 10.1152/jn.00707.2010
7. Gentet, L. J., Stuart, G. J. & Clements, J. D. (2000) Direct Measurement of Specific Membrane Capacitance in Neurons. Biophysical Journal. 79, (1) pp. 314-320. doi: 10.1016/S0006-3495(00)76293-X
8. Harris Lab (APIG). Image-Guided Electrophysiology. Janelia Research Campus. Accessed from https://www.janelia.org/lab/harris-lab-apig/research/activity-tools/image-guided-electrophysiology
9. Kushibiki, T., Okawa, S., Hirasawa, T. & Ishihara, M. (2014) Optogenetics: Novel Tools for Controlling Mammalian Cell Functions with Light. Int. J. Photoenergy. pp 1-10. http://dx.doi.org/10.1155/2014/895039
10. Li, X., Gutierrez, D. V., Hanson, M. G., Han, J., Mark, M. G., Chiel, H., Hegemann, P., Landmesser, L. T. & Herlitze, S. (2005) Fast noninvasive activation and inhibition of neural and network activity by vertebrate rhodopsin and green algae channelrhodopsin, Proc Natl Acad Sci USA. 102, (49), pp.17816–17821. DOI: 10.1073/pnas.0509030102
11. Molecular Devices. (2012) The Axon Guide: Electrophysiology and Biophysics Laboratory Techniques. Third Edition.
12. Ogden, D. & Stanfield, P. (1994). Patch clamp techniques for single channel and whole-cell recording in Microelectrode techniques: the Plymouth workshop handbook, Second Edition. pp 53–78.
13. Prè, P., Nestor, M. W., Sproul, A. A., Jacob, S., Koppensteiner, P., Chinchalongporn, V., Zimmer, M., Yamamoto, A., Noggle, S. A. & Arancio, O. (2014) A Time Course Analysis of the Electrophysiological Properties of Neurons Differentiated from Human Induced Pluripotent Stem Cells (iPSCs). PLOS One. Jul 29;9(7):e103418. doi: 10.1371/journal.pone.0103418
14. Suk, H. J., van Welie, I., Kodandaramaiah, S. B., Allen, B., Forest, C. R., Boyden, E. S. (2017) Closed-Loop Real-Time Imaging Enables Fully Automated Cell-Targeted Patch-Clamp Neural Recording In Vivo. Neuron. 95, (5), p1037–1047.e11. DOI: 10.1016/j.neuron.2017.09.012
15. van Wyk, M., Pielecka-Fortuna, J., Löwel, S. & Kleinlogel, S. (2015) Restoring the ON Switch in Blind Retinas: Opto-mGluR6, a Next-Generation, Cell-Tailored Optogenetic Tool. PLoS Biol. May 7;13(5):e1002143. doi: 10.1371/journal.pbio.1002143
16. Zilberstein, Y., Ewer, J. & Ayali, A. (2006) Neuromodulation of the locust frontal ganglion during the moult: a novel role for insect ecdysis peptides. J. Exp. Biol. 209, pp. 2911-2919. DOI: 10.1242/jeb.02339
17. Novellino, A.. (2010). Recurrence Quantification Analysis of Spontaneous Electrophysiological Activity during Development: Characterization of In Vitro Neuronal Networks Cultured on Multi Electrode Array Chips. Advances in Artificial Intelligence. 2010. 10.1155/2010/209254.
