Introduction
Conventional fluorescence microscopy uses high-intensity light to illuminate the sample but this excites all fluorophores in the light path, not just the plane of interest. The result is that light emitted from outside the focal plane contributes to the image. Confocal microscopy overcomes this problem by using pinholes to selectively collect light only from the plane of interest. However, high-intensity light still penetrates through the entire sample which causes photobleaching and photodamage.
Light Sheet Fluorescence Microscopy (LSFM) or Selective Plane Illumination Microscopy (SPIM) only illuminates the plane of interest which allows us to collect information from a single plane while also minimizing photobleaching and photodamage to the rest of the sample. By eliminating out-of-focus light in this way, lower light intensities can be used to excite the sample which further contributes to the reduction in photobleaching and photodamage, allowing us to image for extended periods of time. More exposures can, therefore, be taken using LSFM than any other form of fluorescence microscopy.
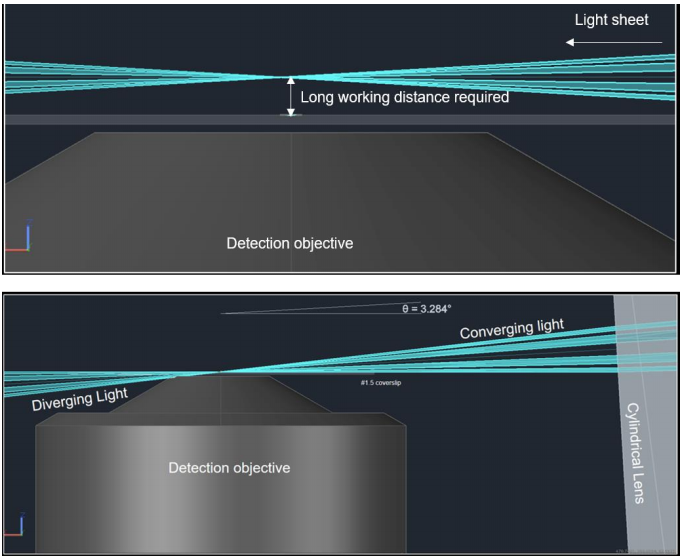
Conventional LSFM is performed with two objectives oriented orthogonally to each other so that one objective introduces the light sheet and the other detects the fluorescence signal. However, this orientation requires the detection objective to be placed slightly away from the sample to prevent the two objectives from colliding in space. Therefore, a long working distance detection objective is necessary which means that high NA, oil-immersion objectives are incompatible with the conventional LSFM design.
This presents a problem for the detection of cellular or subcellular structures that require a high NA detection objective for the superior resolution and light collection efficiency. Imaging these structures is difficult with conventional LSFM and could only be achieved through multi-view SPIM geometries with a 1.2 NA objective and subsequent deconvolution – an incredibly time-consuming and data-heavy process.
Tilt microscopy overcomes this problem by removing the illumination objective and introducing a tilted light sheet through a photomask and cylindrical lens which can be made to converge at the working distance of high NA objectives. In this way, high magnification and high NA (60x, 1.49), oil-immersion objectives can be used to image coverslip-based mounted samples. It can be applied to most existing upright or inverted microscope systems and requires no computational reconstruction to view.
Mizar Tilt Principle
The Tilt was created by Paul Maddox at the University of North Carolina as a way to combine the low photobleaching and photodamage of LSFM with high magnification, high NA objectives for cellular and subcellular imaging. It is manufactured and distributed by Cairn. In conventional LSFM, the position of the objectives results in a light sheet that converges away from the detection objective which necessitates a long working distance detection objective. These objectives typically have low magnification and low NA (Fig.1, Top).
The principle behind Tilt is a technique known as lateral interference tilted excitation (LITE). In this technique, the laser beam is first collimated to a radially symmetric beam of 22 mm diameter. The collimated light is then passed through a photomask and cylindrical lens which focuses the light into a beam approximately shaped like a rectangular prism. The cylindrical lens focuses the beam along one axis to generate a non-diffracting sheet of light which is then tilted so that the bottom of the beam is parallel to the focal plane of the detection objective. The photomask placed before the cylindrical lens creates an interference pattern that elongates the length of the sheet. The light sheet is thereby formed at the working distance of high magnification, high NA lenses (Fig.1, Bot).
Mizar Tilt Advantages
The primary advantage of the Mizar TILT is applying the benefits of LSFM to imaging cellular and subcellular structures. Conventional and confocal microscopy are invaluable tools for cell biology but with light sheet microscopy, far longer acquisitions are possible due to greatly reduced photobleaching and photodamage. Higher NA objectives are also more light efficient than conventional LSFM objectives which means that even lower light intensity can be used, further reducing photobleaching and photodamage. This allows cell biologists to follow processes over much longer timescales than previously possible.
The Mizar Tilt is very versatile in terms of equipment needed. The Mizar Tilt system can be mounted on most upright or inverted microscope systems with any laser lines and camera. This means that anyone with an existing microscope can adapt it into a high-resolution light sheet system. As it is mounted on a microscope, it can also be combined with differential interference contrast microscopy (DIC) or other microscopy tools simultaneously. Furthermore, no special camera modes or extra features are necessary, making the system very simple to implement with existing equipment.
Finally, the Mizar Tilt allows samples to be mounted on coverslips, the preferred mounting modality of cell biologists so no additional technical knowledge of sample preparation is necessary. There is also no need for complex deconvolution of image reconstruction following data acquisition.
Mizar Tilt Camera Choice
Any camera can be used with the Mizar Tit but we believe that the best performance can be achieved with the 95% quantum efficient, back-illuminated scientific CMOS camera, the Photometrics Prime 95B.
The almost perfect, 95% quantum efficient (QE) sensor allows light intensity to be reduced even further while maintaining a high level of signal detection. This is ideal for samples that need to be monitored over very long timescales without photobleaching as well as samples that are particularly sensitive to photodamage. Compared to standard sCMOS devices, the exposure time on the Prime 95B could be reduced by up to four times and still give equivalent detection.
The Prime 95B also has a large field of view (18.66 mm diagonal) and high speed (82 fps, full-frame) expected of CMOS devices. This allows for large samples to be imaged without requiring any stitching. If a larger field of view is required, a version of the Prime 95B with a 25mm diagonal is also available.
The large, 11×11 μm pixels provide additional sensitivity and have a large 80,000e- full well capacity with a low 1.6e- read noise, giving the 95B a very high dynamic range, ideal for performing high contrast imaging. The larger pixels complement high magnification, high NA objectives which allows for high magnification, high-resolution imaging. We would encourage anyone considering using the Mizar Tilt to request a demonstration of the Prime 95B to see the advantages it provides.
Download As PDF
References
Fadero, T. C., Gerbich, T. M., Rana, K., Suzuki, A., DiSalvo, M, Schaefer, K. N., Heppert, J. K., Boothby, T. C., Goldstein, B., Peifer, M., Allbritton, N. L., Gladfelter, A. S., Maddox, A. S. & Maddox, P. S. (2017) LITE microscopy: a technique for high numerical aperture, low photobleaching fluorescence imaging. Preprint. https://doi.org/10.1101/181644
