Prof. Viki Allan
Mr. Daniel Han
Division of Molecular & Cellular Function, The University of Manchester
Background
Prof. Viki Allan is the leader of a group at the University of Manchester that studies cell dynamics, with a particular focus on intracellular transport and the endocytic pathway. Membrane organelles move around cells along microtubule tracks, driven by proteins such as kinesin and cytoplasmic dynein, and this movement of material within a cell is vital for cell function. Prof Allan and the team are studying these intracellular structures through imaging of living cells.
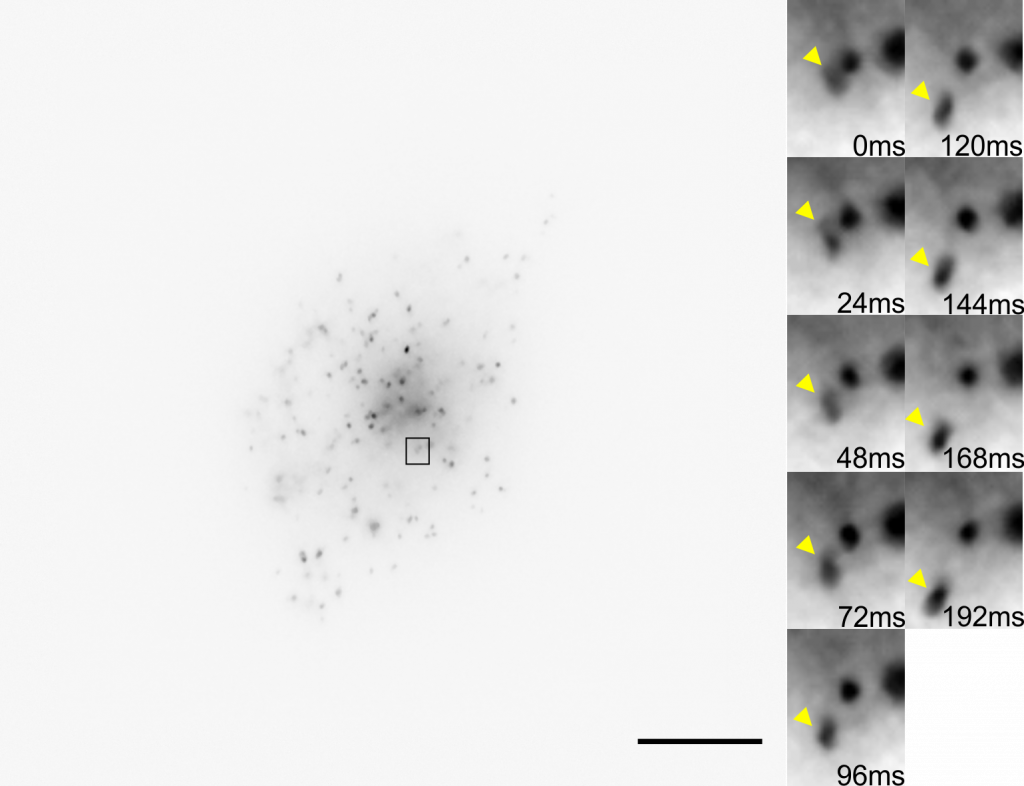
Challenge
There are a number of challenges involved in this imaging research, mainly due to the intracellular cargo and transport networks being very small, having a dim signal, and moving rapidly.
Many intracellular membrane organelles are smaller than the diffraction limit for imaging (~200 nm) and can be difficult to resolve. These samples are labeled with low levels of GFP in order to avoid altering organelle behavior, meaning that they don’t exhibit a bright signal, making detector sensitivity a must. In addition, these transport structures can move at a rate of 8 µm/s (typically ~2-5µm/s), which makes them a challenge to track when imaging at high magnification. In order to track these objects successfully, a large field of view and a high framerate camera would be necessary.
Using a camera with the right pixel size could satisfy the Nyquist criterion and achieve a high resolution at a lower field of view, combined with high sensitivity and high speeds. The choice of camera is important when facing these challenges.
With [the Prime 95B] we can go at faster framerates than with an EMCCD, and we can use full frame to see as much of the cell as possible.
Solution
Even considering the numerous challenges, the Prime 95B sCMOS offers a versatile platform for imaging and has been used by Prof. Allan and the team with great success.
As stated by Prof. Allan: “With [the Prime 95B] we can go at faster framerates than with our EMCCD, and we can use full frame to see as much of the cell as possible.” The Prime 95B is back-illuminated and features a near-perfect 95% peak QE: this and the large 11 µm pixel size make the Prime 95B highly sensitive and a great fit for low-light imaging. In addition, the pixel size is smaller than previous EMCCD solutions, allowing Prof. Allan and group to image at a lower magnification while maintaining a high resolution, therefore increasing the available field of view and improving light throughput. This larger field of view and the high speeds of the Prime 95B result in more efficient particle tracking, imaging as much of the cell as possible. Overall, the Prime 95B met the challenges presented by the sample, resulting in a recent publication (Han et al., 2020).
References
Han D., Korabel N., Chen R., Johnston M., Gavrilova A., Allan V.J., Fedotov S., Waigh T.A. (2020) Deciphering anomalous heterogeneous intracellular transport with neural networks, eLife 9:e52224, https://doi.org/10.7554/eLife.52224

