Dr. Ute Becherer
Department of Cellular Neurophysiology, Saarland University, CPIMM, Homburg
Background
Dr. Ute Becherer studies the regulation of exocytosis and endocytosis using several different model systems, including the release of vesicles containing adrenaline from chromaffin cells (neuroendocrine cells), synaptic transmission in neurons, and the mechanisms of immune cytotoxic T cells. Proteins involved in regulating exocytosis are also present in T cells, and learning more about the processes behind exocytosis is relevant for entire organisms. Dr. Becherer is studying this exocytosis from the molecular scale to entire in vivo animals.
Imaging plays a big part in these experiments, with Dr. Becherer using super-resolution microscopy, TIRF microscopy, confocal microscopy, and widefield imaging. The latter is done with neuronal cells, as stated by Dr. Becherer, “We are looking at a protein called CAPS, which has a role in pain generation, we look at this in the dorsal root ganglion (DRG) sensory neurons in widefield.”
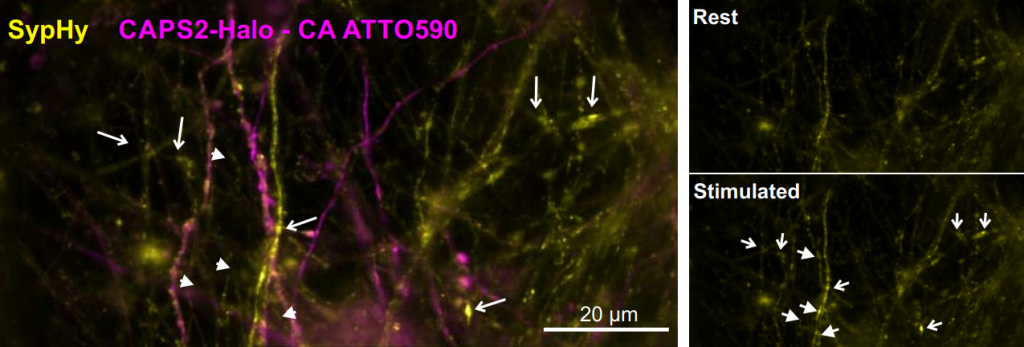
Challenge
As these experiments range across several different imaging techniques, any detectors used need to be flexible and have broad applications. For example, detectors used with the TIRF systems need to be sensitive and fast. Widefield and confocal imaging of cell populations require a large field of view (FOV) in order to capture as many cells as possible and observe as many exocytosis events as possible. Dr. Becherer mentioned that only a portion of these cells are successfully transfected, and a larger FOV allows for a larger sample size.
Some of these systems previously used an EMCCD, but for measuring T cell exocytosis or neuronal synaptic transmission a bigger field of view FOV was required to measure lots of events at once. In addition, Dr. Becherer said, “To really resolve the event and see how large the vesicles were, we needed a smaller pixel size, and the EMCCD pixels were a lot bigger than they are now with the Prime 95B”.
A big plus point of the Prime 95B is the big FOV, the other plus point is the pixel size, coming together with very good sensitivity
Solution
Dr. Becherer is currently using the Prime 95B sCMOS for imaging experiments, making use of the high sensitivity and large FOV, as well as the improved resolution compared to EMCCD due to the smaller 11 μm pixel size. Fluorescence imaging of synaptic transmission was greatly facilitated by the very large field of view provided by the Prime 95B.
By combining the large FOV of the Prime 95B with an iLAS TIRF system, which can image across a larger FOV than classical TIRF, a powerful large field imaging system has been developed.
Dr. Becherer also stated, “I also experienced excellent customer support, and setting up the Prime 95B was easy with PCI. We also upgraded to solid-state drives and more RAM in order to run long acquisitions and collect data.”

