Prof. Enrico Gratton
Founder and Principle Investigator
Laboratory of Fluorescence Dynamics, University of California, Irvine
Background
The lab of Prof. Enrico Gratton at the University of California, Irvine, is interested in the dynamics of the cell interior. The group investigates this using microscopy combined with mathematical approaches such as fluorescence correlation spectroscopy. As the founder and Principle Investigator of the Laboratory of Fluorescence Dynamics, Prof. Gratton’s team has developed numerous fluorescence-based methods to measure diverse cell properties. This includes measurement of the absolute concentrations of molecules within cellular compartments, detection of aggregation of proteins and the detection of barriers to diffusion. Prof. Gratton also developed Globals for Images, a set of software packages to perform this analysis.
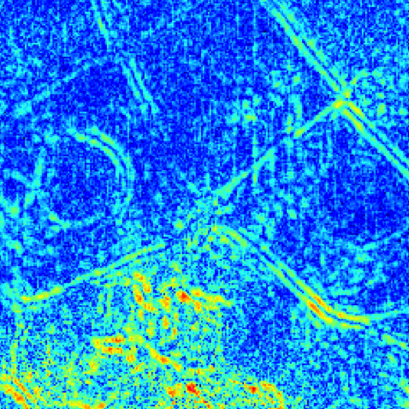
The parallel lines of signal clearly define the boundaries of diffusion at each side of the cell walls. Unlike super-resolution microscopy or other methods, fluctuation correlation spectroscopy images the diffusion itself, rather than the cellular structures which are often not visible.
Time series of 2100 frames over 10.5s, 256×256 pixels, 140nm effective pixel size.
Challenge
To reliably detect the incredibly small fluctuations in image signal required for fluorescence correlation spectroscopy – on the order of 10 electrons of signal – stable and predictable noise characteristics are necessary. Noise is an inherent feature of camera sensors and is corrected to reveal fluorescent fluctuations which are detected and measured. Typical experiments using EMCCD cameras require the noise characteristics, including correlated noise and light-independent pixel variance, to be mapped before every experiment to account for instability in the camera noise over time. After fast, time series fluorescence imaging, the previously mapped noise is removed to detect the variance due to fluorescent fluctuations in each pixel, and allowed mathematical analysis of the signal. With fluctuations in signal being less than 10 electrons in amplitude, high camera sensitivity and being able to accurately correct for the noise are crucial in providing the necessary signal to noise for this analysis.
Prof. Gratton now uses the Prime 95B back illuminated Scientific CMOS camera to take advantage of the stable noise and uncorrelated pixel noise properties.
Solution
Prof. Gratton now uses the Prime 95B back illuminated Scientific CMOS (sCMOS) camera, to take advantage of the stable noise and uncorrelated pixel noise properties. Light-independent pixel noise characteristics are stable over time which means the camera only requires calibration and mapping once, rather than before every experiment.
Prof. Gratton shared, “I didn’t need to account for camera noise in the analyses, unlike when using EMCCDs and other CMOS cameras, which suffer from unstable noise. The reliability and stability of the pixel noise, makes the Prime 95B Scientific CMOS camera an ideal imaging solution for fluorescence correlation spectroscopy methods.” Having predictable and easily modelled pixel to pixel variation produces the highest signal to noise ratio and results in detection of smaller fluctuations than possible when using an EMCCD.

