Professor Andrew Plested
Leibniz Research Institute for Molecular Pharmacology, Humboldt University Berlin
Background
The Plested lab investigates the characterization of receptors relevant to neuronal systems. They apply multiple techniques to image isolated neurons, including a combination of electrophysiology and fluorescence live-cell imaging.
One of their areas of study involves measuring Foerster Resonance Energy Transfer (FRET) upon electrical stimulation of the neurons. They label individual relevant structures and receptors with suitable fluorophores and observe the FRET signal in the dendritic region of cells of interest. This enables them to obtain information about the state, localization, and behavior of channels, and particularly their colocalization in the nanometer range.
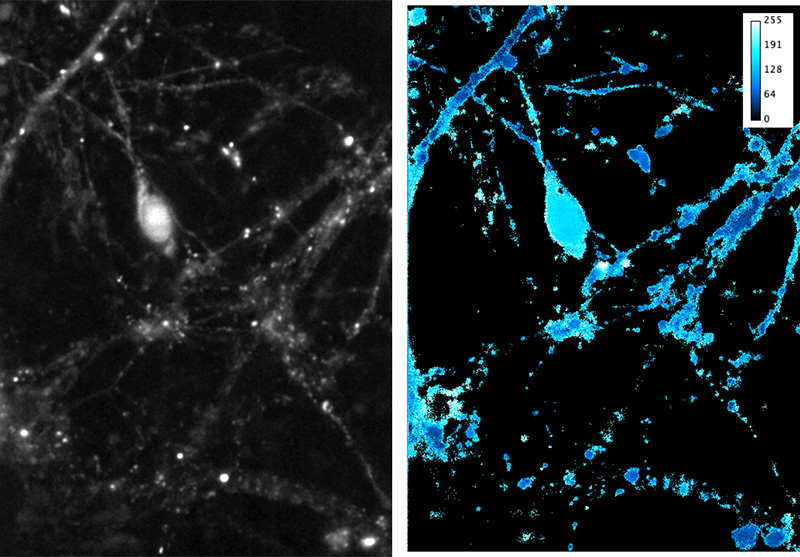
Right Average FRET signal (8-bit, max 255) from 10 frames (100 ms total exposure). Maximum projection was thresholded and used to mask FRET calculation, restricting to cell processes. FRET calculation was done in IGOR Pro. Image manipulation in ImageJ.
Challenge
The individual interactions the group is interested in observing only last for a very brief period of time (10-100 ms). Therefore, to capture the weak signal arising from the energy transfer from a donor fluorophore to an acceptor molecule, very fast imaging is required. This means the number of photons available for detection is very limited and an extremely sensitive imaging device is essential.
Their currently used EMCCD camera – although sensitive enough for detection – is only able to image a very small field of view which makes experimental measurements a time-consuming exercise. FRET measurements are also limited by the 30 ms read time per frame of the EMCCD camera, achieving just 30 fps (corresponding to ~15 fps FRET) at full field. Furthermore, binning had to be used to increase frame rate, reducing spatial resolution.
The Prime 95B lets us look at 5× the FOV at about twice the speed, but with the same sensitivity as our previous EMCCD system.
Solution
The Prime 95B Scientific CMOS camera delivers three critical advantages to Dr. Plested’s experiments: Sensitivity, field of view (FOV) and speed.
First, the relevant power of excitation can be reduced by a factor of 5 – giving a more physiologically-relevant measurement and also reducing artifacts caused by bleaching.
Second, the larger sensor of the Prime 95B resulted in about five times the field of view, dramatically increasing the throughput of cellular regions per experiment.
And finally, along with the larger field of view, the frame rate can also be increased. By imaging at 50 fps (effective FRET measurement at 25 fps, with alternating excitation, see Figure 1) the data is much more relevant to synaptic transmission (timescale of interest 10-100 ms). This means that their measurements are now limited by probe properties, not the camera.
The Prime 95B gave the Plested lab an improved way to obtain their data, switching from essentially single object imaging to a multiple neurite view, yielding more detailed insight into synaptic transmission.

