Prof. Kirill Volynski
Institute of Neurology, University College London
Background
The Volynski group are primarily interested in understanding cellular regulation of synaptic release of neurotransmitters which forms the basis of communication among neurons in the brain.
The Volynski group uses fluorescent probes, such as vesicular release sensor synaptophysin-pHluorin (sypHy). They visualize synaptic transmission at the level of single synapses in order to understand the basic mechanisms of transmitter release and also the cellular mechanisms of synaptopathies – paroxysmal neurological disorders caused by mutations in synaptic proteins. These disorders include some forms of epilepsy, migraine and ataxias.
The sypHy probe consists of a pH-sensitive GFP variant fused with the vesicular membrane protein synaptophysin. sypHy is widely used to image synaptic vesicle exo- and endocytosis. At rest, the sypHy fluorescence inside of a synaptic vesicle is quenched by the acidic vesicular pH. Upon synaptic vesicle exocytosis, caused by neuronal spiking, the sypHy probe becomes exposed to the extracellular medium with neutral pH, which leads to an increase in its fluorescence. Quantification of the fluorescence signal provides a means to monitor the response of individual synaptic boutons to different stimuli, thus providing a readout for the effects of disease-linked mutation effects on synaptic function.
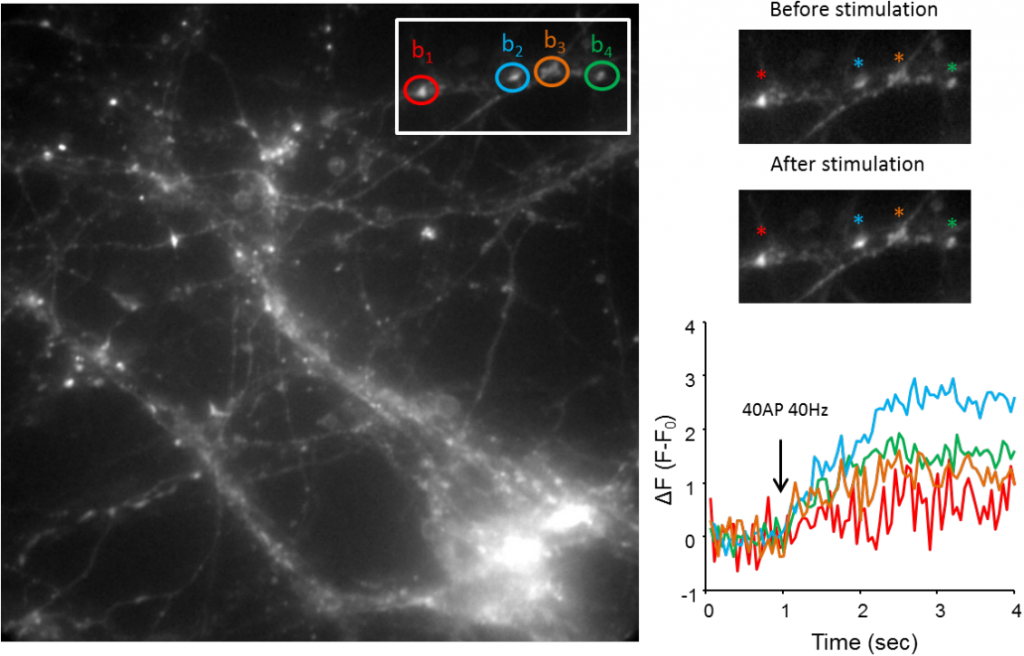
Challenge
Prof. Volynski was initially capturing images with a 512×512 EMCCD camera, however, imaging routines often consisted of several seconds which led to problems with drift in the temperature of the EMCCD sensor accompanied by a drift in the camera gain and ultimately the signal. Although this can be corrected posthoc it is non-trivial and complicates the analysis.
In addition to this, Prof. Volynski also wanted to image at higher speeds to capture the fluorescence associated with neurotransmitter release as well as synchronizing the camera with electrophysiological traces. However, when using the EMCCD, speed was limited to 30 fps and often frames were dropped randomly, which led to difficulties in the acquisitions and syncing.
We switched to the Teledyne Photometrics Prime 95B and now have very stable responses, the camera works faster, and the SNR is very comparable to EMCCDs.
Solution
The Volynski group is now using the Teledyne Photometrics Prime 95B to image synaptic transmission. Professor Volynski told us that “Stability and speed were the key issues here, and once we started using the Prime 95B the problem of instability totally disappeared. We switched to the Teledyne Photometrics Prime 95B and now have very stable responses, the camera works faster, and the SNR is very comparable to EMCCDs” He shared that, “We can acquire the same region of interest faster with the Prime 95B compared to the EMCCD.”
Professor Volynski went on to say that, “Now, we have a very robust protocol for detecting synaptic responses both at the level of single boutons and populations of synapses which provided us with a reliable and fast method for testing the synaptic effects of the disease-linked mutations”.

