Mr. Marco Schnieder, Prof. Jürgen Klingauf
Institute of Medical Physics and Biophysics, University of Münster, Germany
Background
Marco Schnieder is a PhD student in the group of Prof. Klingauf, whose research focuses on neuroscience, mainly the physiology of synaptic transmission, and the mechanisms of synaptic vesicle recycling, in particular endocytosis.
The cellular and protein machinery involved in synaptic transmission is investigated in the Klingauf Lab using extremely sensitive high- and super-resolution imaging techniques on cultures of living neurons. Fluorescence microscopy is combined with electrophysiology, where all neurons are excited by electrodes simultaneously in order to trigger action potentials, at which point synaptic transmission of vesicles between neurons can be observed.
Mr. Schnieder uses pH-sensitive probes such as pHluorin to track synaptic vesicles at both the pre- and post-synaptic neurons, as well as observing protein machinery involved in synaptic vesicle fusion, such as the SNARE protein complexes. This is all done with live neuronal cultures and imaged with TIRF.
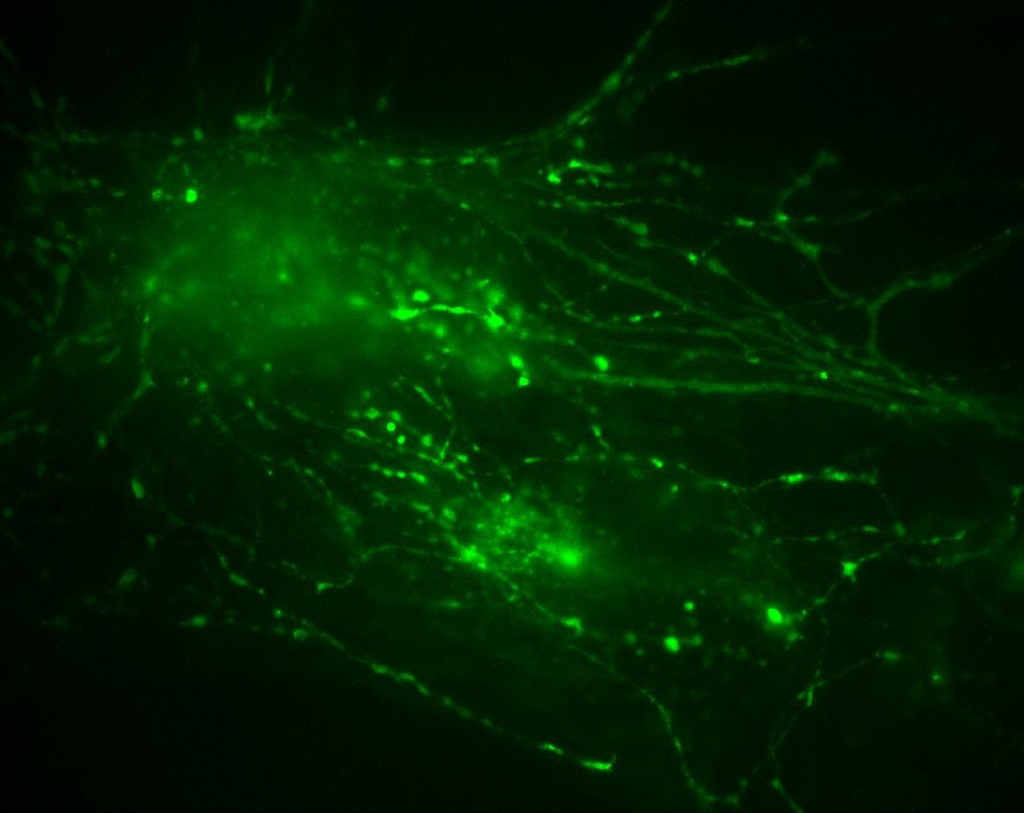


Figure 1: Neuronal cells imaged using the Prime 95B sCMOS camera. Image is part of a stack where stimulation is applied, two regions of interest are shown as part of the full stack, where endocytosis and synaptic vesicles can be observed in motion through the cell.
Challenge
The first challenge was the sample size, as described by Mr. Schnieder, “The vesicles and endocytosis machinery are so small, far beyond the classical diffraction limit at ~40 nm. Nevertheless, we would like to observe them in living cells. While electron microscopy is suitable for high resolution, it is not suitable for imaging living cells, this is why we use fluorescence microscopy.”
As well as requiring high spatial resolution, this application also benefits from high temporal resolution, as the events occurred on a second/sub-second scale. Other techniques such as STORM were available but were not used due to the speed limitations.
Sensitivity is also vital for quantitative research; one aim is to determine the number of pHluorin molecules within vesicles by the fluorescence intensity. The signal flux is very small, meaning a highly sensitive camera with high signal collection but low read noise is necessary.
The [Prime 95B] camera has the best quantum efficiency, this is decisive when you have both temporal and spatial resolution thresholds, this was the best camera for the job.
Solution
The Prime 95B sCMOS is an ideal solution for this application, as outlined by Mr. Schnieder, “The Prime 95B is really advantageous because it’s a CMOS, so it doesn’t enhance the noise of the measurement as EMCCDs do. Furthermore, the 95% quantum efficiency is really good for these dense culture measurements as our change in fluorescence intensity is low… there is a positive difference in the quality of the data compared to our other CMOS.”
The 95% QE, large pixel, and low read noise of the Prie 95B result in EMCCD-like levels of sensitivity, while also maintaining CMOS advantages of a larger field of view and higher speed. With the low noise, intensity changes can be identified in order to perform quantitative analysis while maintaining high image quality.
Regarding the pHluorin imaging, the lab further increased its confidence in being able to resolve numbers of present and signal-contributing pHluorin molecules during exo- and endocytotic events. This enables a more reliable and more quantitative analysis.

