Prof. Thomas Etheridge
Antony Carr Group, Genome Damage and Stability Centre, University of Sussex
Background
The Carr Lab at the Genome Damage and Stability Centre, University of Sussex, investigates DNA metabolism processes such as DNA replication and repair. They are interested in the challenges cells face during DNA replication and the cellular processes that help the cell overcome replication fork stalling or collapse. To study this, they focus on the behaviors and interactions of individual proteins involved in DNA replication in both fission yeast and human cells.
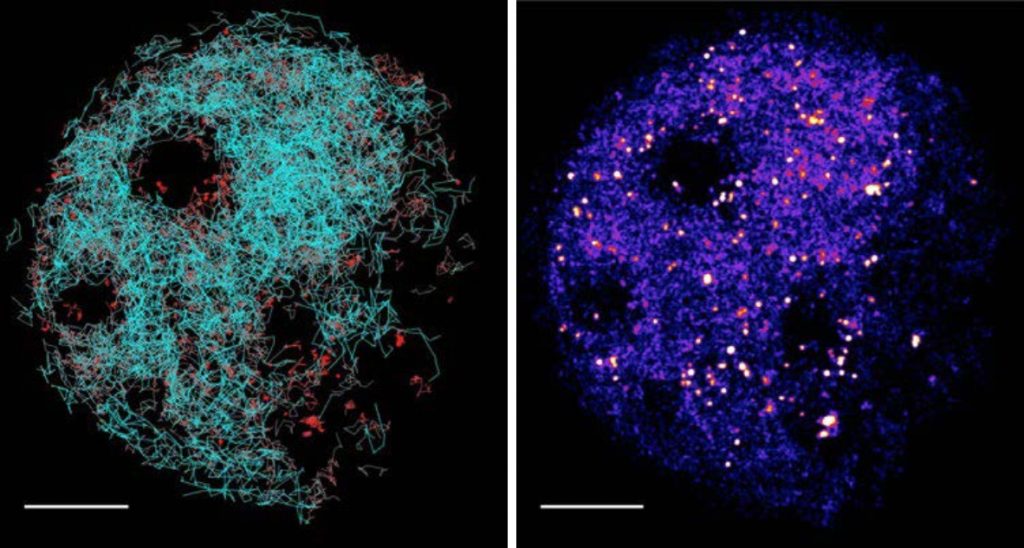
Left: Superimposed tracks of detected molecules. Red = DNA-bound, static molecule. Cyan = freely diffusing complexes.
Right: Localization map of complexes. Hot spots indicating locations of DNA bound molecules.
Challenge
One approach used to examine the DNA association of proteins is to break open the cell, extract the proteins and perform western blotting. However, this approach reveals nothing of the dynamics of association, and destroys cells in the process.
A more demanding alternative is to use an imaging technique capable of single protein resolution. To this end, Dr. Etheridge uses Single Molecule Tracking PALM to observe the association of proteins with the DNA in real time, non-invasively, in both yeast cells and human cells. Single fluorescent molecules move quickly and give off very little light so this technique requires high speed and the highest sensitivity.
The fluorescent proteins used in the yeast cells are very dim and, until now, Dr. Etheridge has been using an EMCCD camera to observe them. However, the field of view given by his EMCCD camera is quite small. For human cells, brighter synthetic dyes can be used so dynamics can be observed with better temporal resolution. Unfortunately, the lower speed of his EMCCD camera doesn’t allow for this.
Compared to the EMCCD, the Prime 95B gives us a field of view large enough for our most demanding experiments as well as faster frame rates.
Solution
The back illuminated Prime 95B scientific CMOS camera has the speed and field of view advantages expected of CMOS cameras, but with the sensitivity to rival or beat EMCCDs. This gives researchers using single molecule techniques unprecedented low-light detection and temporal resolution.
Dr. Etheridge shared, “To image the demanding yeast cells with our EMCCD, we were having to do multiple experiments on different areas of the sample to generate enough data for it to be worth processing. With the field of view of the Prime 95B, we can gather enough cells in the field of view to process in a single shot. In the human cell system, using the brighter dyes, we can image our favorite proteins at much faster speeds.”

