Prof. Keith Weninger
Department of Physics, North Carolina State University, US
Background
The lab of Prof. Keith Weninger develops single-molecule fluorescence methods to study biomolecular systems, with a particular focus on FRET to study proteins involved in DNA mismatch repair. Prof. Weninger further explained his research, “I do single-molecule FRET experiments on tethered DNA molecules with surface-immobilized TIRF microscopy.”
“Most of our lab is focused on DNA mismatch repair. After copying DNA, the DNA polymerase has an error rate, and a set of proteins follow behind and proofread, repairing any base-base mismatches. DNA polymerase makes a mistake one in every million bases, and the proofreaders improve this by a factor of a thousand, resulting in one in a billion errors from DNA copying in cells. We are interested in these proteins and how they work, and when they don’t work right it’s associated with various cancer phenomena.”
“We do different things, such as build short DNA molecules with mismatches and tether them to a surface and flow the proteins over them. Or we can put a polystyrene bead onto tethered DNA to see the motion and range. For our FRET imaging, we can put FRET fluorophores on the DNA, protein or one on each.”
Challenge
This format of single-molecule microscopy can be highly challenging, due to the combination of a very low signal level and the need for high-speed imaging in order to capture molecular dynamics. Prof. Weninger described his need for speed, “In every experiment we want to push temporal resolution, we basically always work at the limits of technology and so we want to image as fast as we can to capture conformational dynamics and interaction, using sub-millisecond exposures. There are also a variety of different timescales so we need to be flexible.”
“We are at a very low signal, photon-counting level as we are doing single molecule fluorescence. We can increase the intensity of the light source but this can result in bleaching, so we also need good triggering of experiments to minimize bleaching too.”
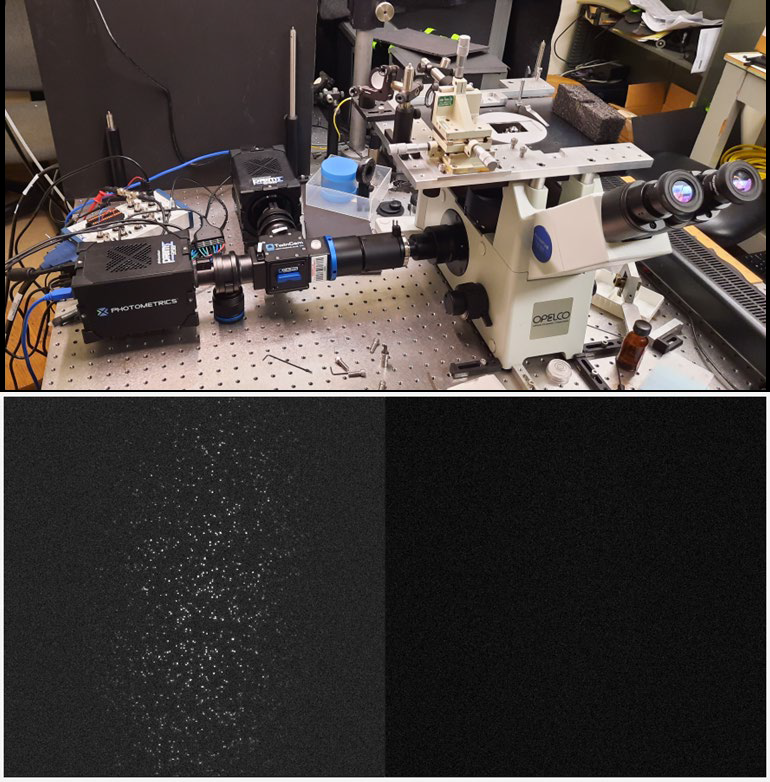
Our two Prime 95Bs produce comparable data to our previous EMCCDs, only now we can image much faster!
Solution
The Prime 95B sCMOS is an ideal solution for high sensitivity single-molecule microscopy, featuring near-perfect 95% signal collection thanks to back-illumination, low noise levels, along with a large 11 μm ideal for high magnification molecular imaging work.
Prof. Weninger described his imaging experiences, “We set up two Prime 95Bs on a TwinCam and they produce comparable data to our previous EMCCDs. We were using them for slow imaging but now we can go fast. We can also use the TwinCam for polarization.”
The use of two cameras allows for high-speed, high-throughput FRET imaging, with specifications well matched for a highly sensitive TIRF imaging system.

