Introduction
An enormous amount of life science research involves imaging cells/tissues of all kinds. There are two main paradigms when imaging cells and tissues: fixed or live.
Live vs Fixed
A ‘fixed’ cell is a cell that is preserved in a state that is as close to “life-like” as possible. The cells die during this process, but their shape and contents are mostly preserved for imaging purposes, and further preparation steps are far easier to perform on fixed cells than live cells. Unfortunately, chemical and physical fixation processes (such as preserving in formaldehyde) irreversibly change the cell composition and appearance, but broad patterns, networks, proteins and DNA can be largely conserved. Cells can also be broken open during fixing, which allows antibodies and chemicals inside the cell for greater ease of imaging.
A large number of images of cells we see today are from cells that are grown on glass, then fixed, prepared/stained and finally sandwiched between a coverslip and sealed, as seen in Fig.1. This allows these cells to be imaged in the same conditions for potentially months and years, essentially freezing the cells in time and allowing for detailed analysis. However, these images are not representative of cell behavior and characteristics in a living system, which is more biologically relevant data.
Live cell imaging is just as it sounds, the cells are imaged while they are alive, usually in their growth medium. Unlike fixed cells, which are representative of static cells that never change, live cells are representative of dynamic cells that move and change over time. This means that imaging over time (termed ‘time-lapse’) is very important for live cell imaging. However, imaging complex 3D objects such as cells or tissues over time results in four dimensions of imaging and a whole host of new issues. A major issue is that of contrast and staining.
Cells and the smaller structures inside cells are mostly water and are therefore transparent. Imaging these bags of water on a glass/clear plastic background results in images that don’t contain a lot of information, and it is vital to have some sort of contrast or dye/stain that will all the cell components to be visualized. For fixed cells, you can simply add the staining agent as the cells are dead and fixed and won’t be affected. For live cells, there is an issue of toxicity and staining a live cell usually results in cell death.
Due to these issues with staining, most live cell imaging was brightfield, just using light to illuminate what little could be observed. It wasn’t until the invention of phase contrast microscopy in the 1940s that live cell imaging really took off, due to the ability to better observe live cells without the use of a contrast/staining agent.
Phase-Contrast Microscopy
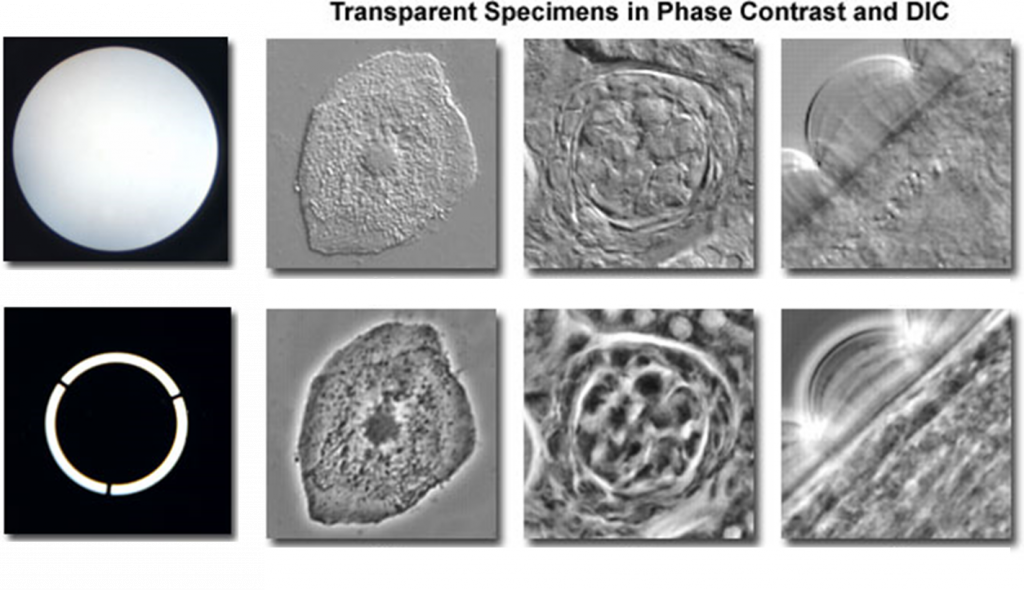
When light travels through a dense medium (water, oil, glass, etc) it decreases in speed. After exiting the dense medium, the light is slightly offset in phase compared to the light that did not pass through anything dense. This is known as phase shift. If the phase-shifted light meets the normal light they will almost cancel each other out (destructive interference), resulting in much lower light intensity at the point where they meet.
Phase-contrast microscopy uses phase shift to modify the intensity of light. Samples are illuminated with light that has passed through a diaphragm, resulting in a ring of light. This ring of light passes through the sample (which is denser than the surrounding air) and is phase-shifted, with the light refracting in different directions depending on the thickness of the part of the sample they passed through. Most biological samples are uneven in thickness and will result in a variety of different refractions. This refracted phase-shifted light passes through a phase ring, made up of an inner and outer section. If refracted light passes through the outer section of the ring it is further phase-shifted and appears as dark. If refracted light passes through the inner section of the ring it appears as bright. In this manner, different parts of the cell are bright or dark depending on their thickness, which allows for much better contrast in the image. This improvement over brightfield can be seen in Fig.2.
Improvements in camera technology, pixel density and sensitivity have led to advances in phase contrast and the development of quantitative phase contrast. Images taken with quantitative phase-contrast technology can be automatically processed to extract huge amounts of information from dynamic cells over time. Images can also be taken at different focal planes from just one exposure, allowing for better 3D imaging, especially with rotational scanning for high-resolution 3D images of live cells over time. These advances allow for live cells to be imaged with far better analysis.
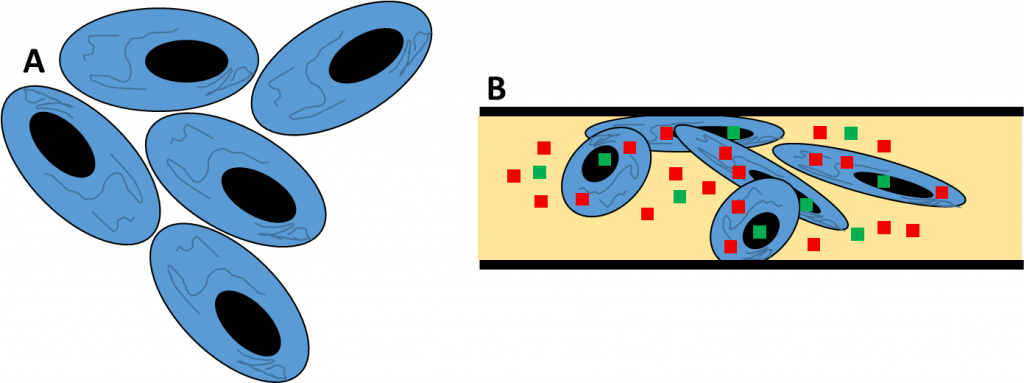
Live Fluorescence Microscopy
If live cells cannot be stained with fluorescent dyes due to toxicity issues, can live cells be imaged with fluorescent microscopy? Phase-contrast cannot observe specific proteins and cellular components like fluorescent microscopy can, limiting the range of experiments that can be performed.
The solution is fluorescent proteins (FPs). The most well-known of these is the green fluorescent protein (GFP), which is outlined in a separate short article titled ‘What is GFP?’ A variety of different colors of FP allow for live fluorescent experiments that are as in-depth and customizable as fixed cell fluorescent imaging. By inserting the gene for GFP directly into the cell genome, genetically encoded FPs allow for non-invasive fluorescent markers to be attached to any protein or cellular component for each imaging, even through several generations as the FP is often heritable. Live fluorescent imaging with multiple FPs can be seen in Fig.3.
While fluorescent live imaging was initially uncertain, with the introduction of FPs it is feasible and simple to do detailed time-lapse imaging of cells with fluorescent labels, opening up the field of the live cell imaging.

Cameras For Live Cell Imaging
When selecting a scientific camera for detection, the desired factors of the camera are highly variable depending on the needs of the sample. If the sample is not fast-moving and requires long exposures for time-lapse, a CCD would be a good choice, but if the sample is fast-moving (such as an organelle within a cell), in low light and needing low exposure times, researchers often use back-illuminated sCMOS cameras. With a wide range of detectors available, ensure that the resolution, sensitivity, speed, and field of view of the camera is matched to the application.
Summary
Overall, live cell imaging represents a powerful alternative to fixed cell imaging, and with recent advances and technologies such as quantitative phase-contrast and live fluorescence, more researchers are imaging their cells live in order to get the most biologically relevant data.
