Introduction
Light sheet is one of the fastest growing fields in microscopy due to the low cost, flexibility and fast 3D imaging of large samples. In other microscopy techniques, the illumination and imaging systems use the same light path and on the same axis. In light sheet, the illumination beam is perpendicular to the imaging system, creating a sheet of light through a 2D plane of the sample, as seen in Fig.1. For more detailed information about light sheet as a whole, please refer to our article on light sheet imaging.
The light sheet imaging setup has multiple benefits. Firstly, a single 2D plane of the sample can be imaged quickly, meaning fast dynamic processes can be resolved in live samples. Secondly, only one plane is being excited, meaning that there is far less background out-of-focus light (when compared to widefield and confocal) emitted, which improves signal.
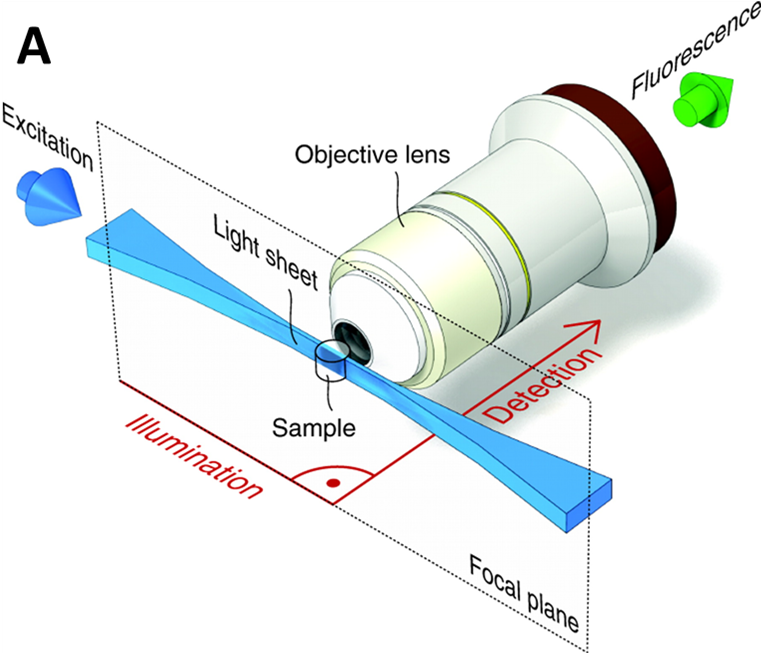
By rotating the sample though a light sheet, 3D images of large samples can be taken over time (resulting in 4D images). While light sheet can take non‑invasive 4D images at cellular resolutions, it is limited by the diffraction limit of light and struggles to image samples much smaller than ~200 nm, such as sub-cellular imaging. Conventional light sheets are too thick to image below cellular resolutions, by developing a technique with much thinner light sheets, smaller samples can be imaged at higher resolutions. This is the principle of lattice light sheet.
Lattice Light Sheet
Lattice light sheet is a combination of several concepts from different microscopy techniques, developed by Eric Betzig (2014). Lattice light sheet involves techniques from light sheet microscopy super-resolution structured illumination microscopy (SIM) and Bessel beams. ‘Super‑resolution’ is an umbrella term for any technique that can break through the diffraction limit of light and image samples smaller than ~200 nm in X and Y. SIM refers to using a structured pattern of light (rather than a single point of light) in order to image a sample at high speed and at super-resolution levels. Bessel beams are outlined in more detail in the next section.
Bessel Beams
Due to the way that light diffracts over a distance, even high-intensity lasers cannot maintain a small spot size over long distances. This is due to the diffraction limit of light, and how samples smaller than 200 nm challenging to resolve. One way to ensure that laser spot size remains small is to use wave optics to shape beams in order to make ‘non-diffractive’ beams. While using a normal spherical lens produces a standard Gaussian beam, using a conical lens (also known as an axicon) results in a central spot surrounded by lower intensity concentric circles, known as a Bessel beam, see Fig.2.
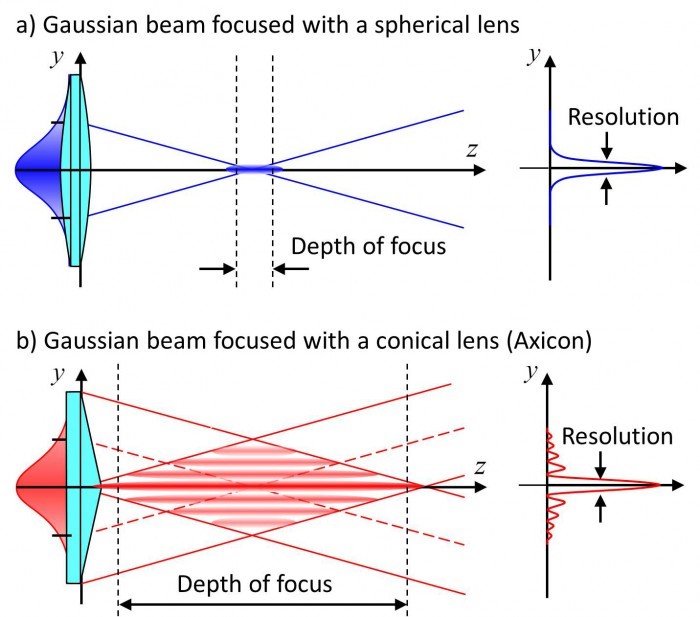
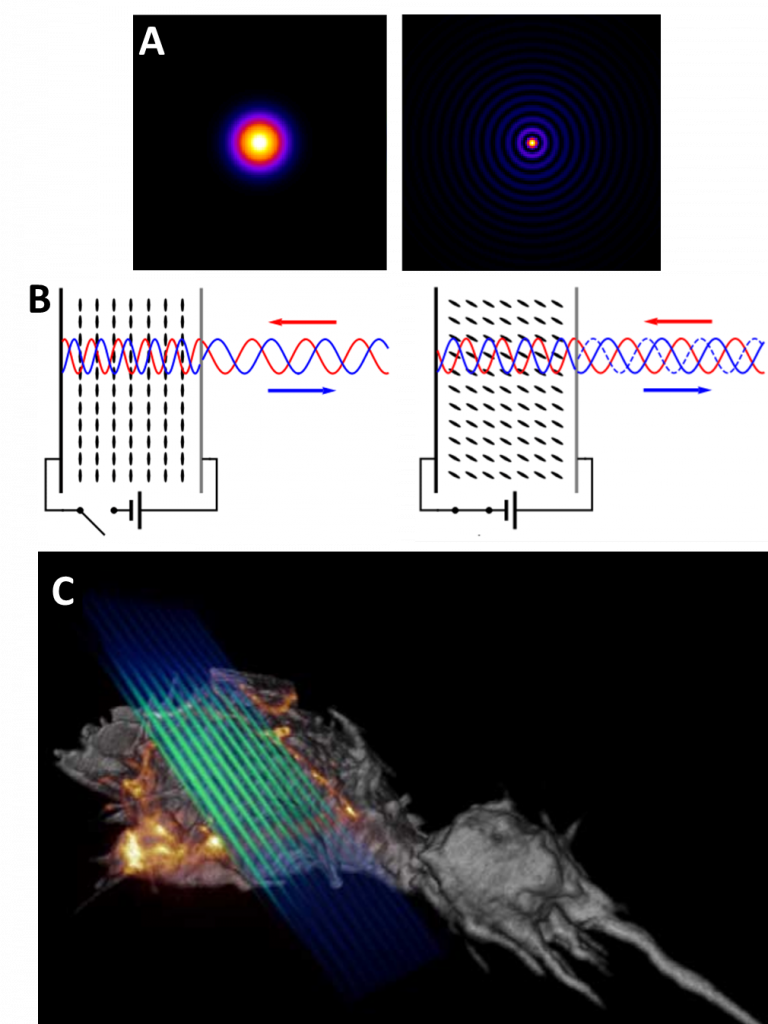
Unlike Gaussian beams (formed of a single point), the central point of a Bessel beam does not diffract as the laser travels, remaining the same size. In addition, if the central point of the beam is blocked, the laser will ‘self-heal’ using the rings that were not blocked, and re-construct on the other side of the blockage via interference. See Fig.4A for more.
Lattice Light Sheet Theory
As described by Eric Betzig, lattice light sheet creates ultrathin light sheets from 2D optical lattices (see Fig.3) that allows for 4D imaging of hundreds of samples at sub-second intervals and super-resolution levels. In essence, lattice light sheet is super-resolution light sheet, performing non-invasive imaging on samples below ~200 nm in size.

In a lattice light sheet microscope, the sample is illuminated perpendicularly from the fluorescence detection, the same as standard light sheet (see Fig.1). The difference in lattice is how the light sheet is optically manipulated. A Bessel beam light sheet is projected onto a spatial light modulator (SLM), a device that can modify characteristics of the light sheet, changing the waveform and introducing a structured pattern (as seen in Fig.4B). The resulting light pattern is filtered and uses destructive interference to reduce the intensity of the outer circles of the Bessel light sheet, resulting in a patterned light sheet that is non-diffractive. This patterned Bessel light sheet is extremely thin due to the interfering patterns, similar to how patterns interact in SIM. This ultrathin light sheet is transmitted through live biological samples (usually cells), as seen in Fig.4C.
For more detailed information on the generation of the lattice light sheet, refer to the original paper from Eric Betzig and colleagues (Chen et al. 2014).
Despite the fact that lattice light sheet involves illumination samples with several beams, it features less photodamage as the lattice has overall less intensity than a standard light sheet. This allows for longer exposure times and time-lapses with lattice light sheet, which when combined with the potential for extremely fast capture rates (200-1000 image planes per second) and super‑resolution levels of detail, it makes lattice light sheet a cutting-edge microscopy technique that presents a powerful platform for imaging biological samples.
Examples
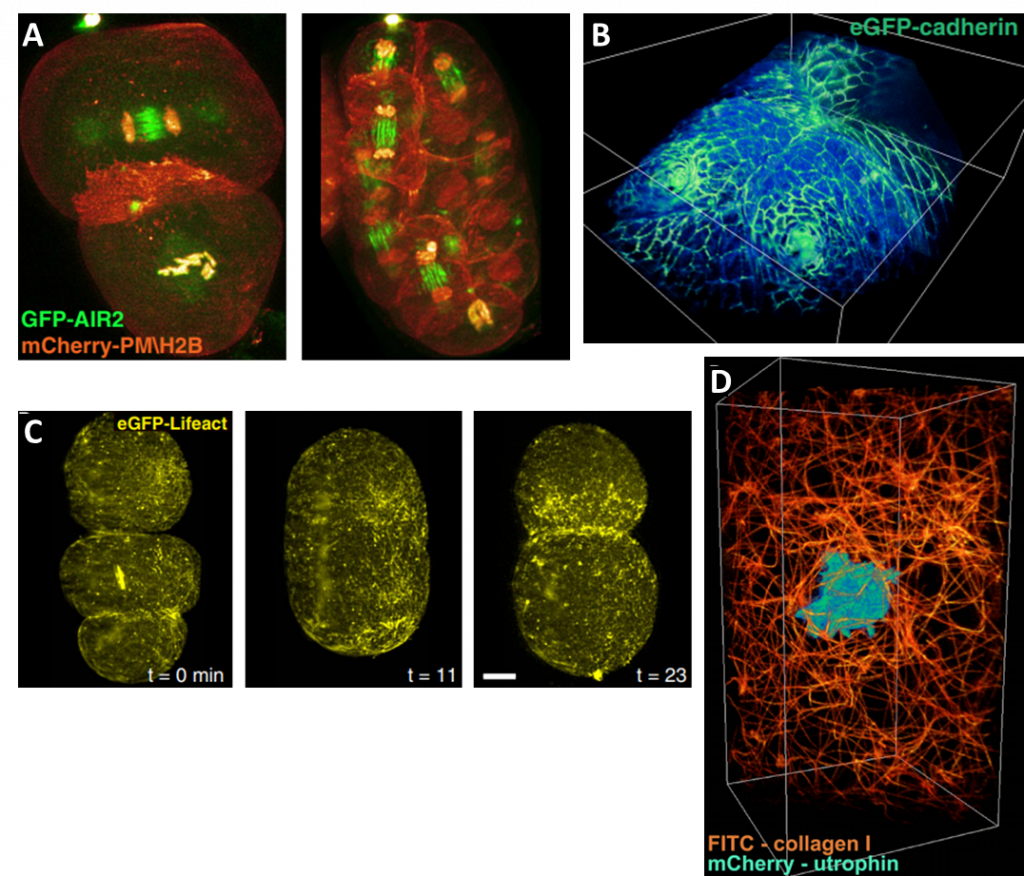
Comparison images from lattice light sheet can be seen in Fig.5, with a selection of full lattice light sheet images seen in Fig.6.
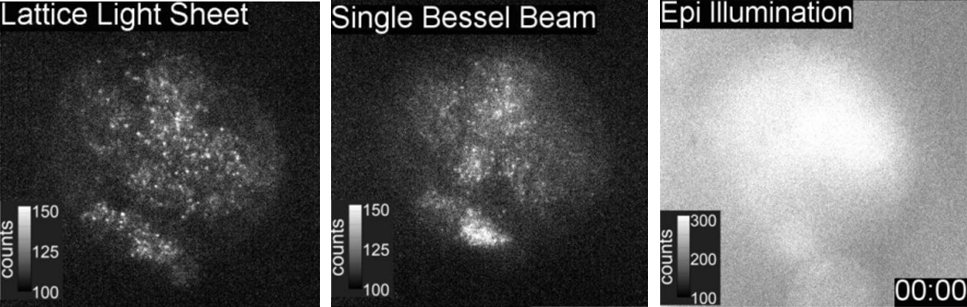
Images from the time-lapse seen in Fig.5 shows the improvement of a lattice light sheet compared to a scanning Bessel beam, with a greater resolution indicating the advantages of making thinner light sheets.
Cameras
As lattice light sheet is a super-resolution technique, suitable detector scientific cameras would have high resolution and small pixels, along with a high quantum efficiency (QE) in order to maximize sensitivity at this resolution level. In addition, lattice light sheet can image at high speeds, so a camera with a high framerate would be necessary in order to not limit the system. The low read noise of sCMOS cameras would also be suitable for quantitative images with a good signal-to-noise ratio.
Summary
While light sheet is an established and popular technique it is stymied by the diffraction limit of light and struggles to define samples smaller than 200 nm. Conventional light sheets are too thick to image below cellular resolutions, new techniques such as lattice light sheet can form much thinner light sheets and image smaller samples at higher resolutions. These thinner light sheets are formed using ‘non-diffractive’ Bessel beams, and allow light sheet to break through the diffraction limit of light.
References
B.-C. Chen, W. R. Legant, K. Wang, L. Shao, D.E. Milkie, M.W. Davidson, C. Janetopoulos, X.S. Wu, J.A. Hammer, Z. Liu, B.P. English, Y. Mimori-Kiyosue, D.P. Romero, A.T. Ritter, J. Lippincott-Schwartz, L. Fritz-Laylin, R.D. Mullins, D.M. Mitchell, J.N. Bembenek, A.C. Reymann, R. Böhme, S.W. Grill, J.T. Wang, G. Seydoux, U.S. Tulu, D.P. Kiehart, E. Betzig (2014) Lattice light-sheet microscopy: Imaging molecules to embryos at high spatiotemporal resolution, Science 346, 1257998.
