Introduction
Total internal reflection (TIR) can occur when light is moving from one medium to a different medium e.g. air to glass, glass to water, water to oil. At certain angles, rather than being refracted into the new medium, the light is reflected back into the medium it came from. TIR only occurs at certain angles, as demonstrated in Figure 1.

In the mid-1950s, E.J. Ambrose had the idea to use TIR to image cells that were adhered to a surface (Ambrose 1956), an idea that was expanded into total internal reflection fluorescence (TIRF) microscopy by Daniel Axelrod in 1981 (Axelrod, 1981).
Epifluorescence vs TIRF Microscopy
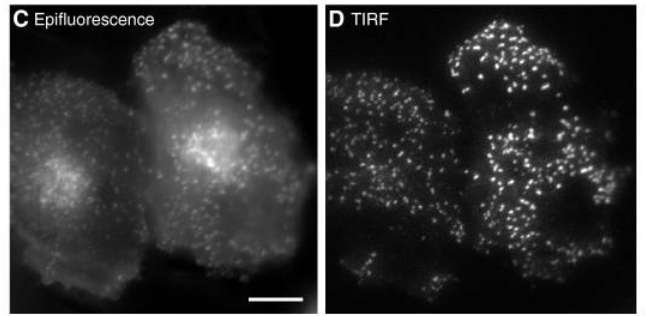
Traditional widefield fluorescent microscopy involves illuminating and exciting the whole sample, with light penetrating more than 1 mm into the sample. However, because the whole sample is illuminated at once emission light is received from the regions of interest and all other regions, meaning that out‑of-focus light emission interferes with the signal and introduces substantial background fluorescence and a poor quality image. The quality of widefield images compared to TIRF can be seen in Figure 2.
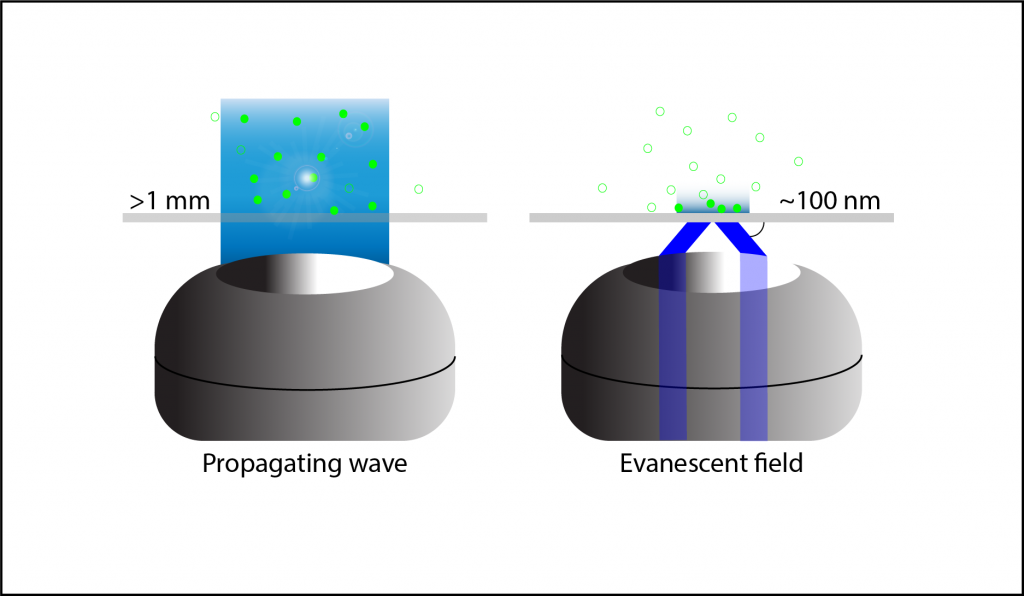
Thanks to the small optical section that is illuminated at the slide interface, there is far less out‑of‑focus light, a better signal, lower light intensity and higher resolution compared to widefield fluorescence, a comparison seen in Figure 3. TIRF is particularly favorable for live cell imaging as low light levels mean the cells are not at risk of phototoxic damage, and for studying areas of the sample close to the surface such as the surface of cell membranes.
Further Reading
Want to learn more? For a full explanation of TIRF microscopy, see our extended application note.
TIRF can also be successfully combined with many other techniques such as FRET, SIM, PALM and STORM as well as other imaging modalities such as spinning spot TIRF. The basics of these techniques are covered in our extended application note but more information can be found in the papers referenced below.
References
Deng, Y., & Asbury, C. L. (2017). Simultaneous Manipulation and Super-Resolution Fluorescence Imaging of Individual Kinetochores Coupled to Microtubule Tips. Methods in Molecular Biology (Clifton, N.J.), 1486, 437–467. http://doi.org/10.1007/978-1-4939-6421-5_17
Ellefsen, K. L., Dynes, J. L., & Parker, I. (2015). Spinning-Spot Shadowless TIRF Microscopy. PLoS ONE, 10(8), e0136055. http://doi.org/10.1371/journal.pone.0136055
Fish, K. N. (2009). Total Internal Reflection Fluorescence (TIRF) Microscopy. Current Protocols in Cytometry / Editorial Board, J. Paul Robinson, Managing Editor … [et Al.], 0 12, Unit12.18. http://doi.org/10.1002/0471142956.cy1218s50
Boulanger, J., Gueudry, C., Münch, D., Cinquin, B., Paul-Gilloteaux, P., Bardin, S., … Salamero, J. (2014). Fast high-resolution 3D total internal reflection fluorescence microscopy by incidence angle scanning and azimuthal averaging. Proceedings of the National Academy of Sciences of the United States of America, 111(48), 17164–17169. http://doi.org/10.1073/pnas.1414106111
Mattheyses, A. L., Simon, S. M. & Rappoport, J. Z. (2010) Imaging with total internal reflection fluorescence microscopy for the cell biologist. J Cell Sci. Nov 1; 123(21): 3621–3628. doi: 10.1242/jcs.056218
Raab, M., Jusuk, I., Molle, J., Buhr, E., Bodermann, B., Bergmann, D., … Tinnefeld, P. (2018). Using DNA origami nanorulers as traceable distance measurement standards and nanoscopic benchmark structures. Scientific Reports, 8, 1780. http://doi.org/10.1038/s41598-018-19905-x
Ramachandran, S., Cohen, D. A., Quist, A. P., & Lal, R. (2013). High performance, LED powered, waveguide based total internal reflection microscopy. Scientific Reports, 3, 2133. http://doi.org/10.1038/srep02133
Ross, S.T., Schwartz S., Fellers, T. J., Davidson, M. W., (2017) Total Internal Reflection Fluorescence (TIRF) Microscopy. Retrieved from https://www.microscopyu.com
Scott, B. L., Sochacki, K. A., Low-Nam, S. T., Bailey, E. M., Luu, Q., Hor, A., … Hoppe, A. D. (2018). Membrane bending occurs at all stages of clathrin-coat assembly and defines endocytic dynamics. Nature Communications, 9, 419. http://doi.org/10.1038/s41467-018-02818-8
Tabor, A., Möller, D., Hübner, H., Kornhuber, J., & Gmeiner, P. (2017). Visualization of ligand-induced dopamine D2S and D2L receptor internalization by TIRF microscopy. Scientific Reports, 7, 10894. http://doi.org/10.1038/s41598-017-11436-1
Thompson, N. L., Burghardt, T. P., & Axelrod, D. (1981). Measuring surface dynamics of biomolecules by total internal reflection fluorescence with photobleaching recovery or correlation spectroscopy. Biophysical Journal, 33(3), 435–454.
Weigel, A. V., Tamkun, M. M., & Krapf, D. (2013). Quantifying the dynamic interactions between a clathrin-coated pit and cargo molecules. Proceedings of the National Academy of Sciences of the United States of America, 110(48), E4591–E4600. http://doi.org/10.1073/pnas.1315202110
Young, L. J., Ströhl, F., & Kaminski, C. F. (2016). A Guide to Structured Illumination TIRF Microscopy at High Speed with Multiple Colors. Journal of Visualized Experiments : JoVE, (111), 53988. Advance online publication. http://doi.org/10.3791/53988
