Introduction
Typical fluorescence microscopy involves illuminating the entire sample and detecting the resulting fluorescence. Illuminating and detecting from the entire sample includes collection of out-of-focus light above and below the focal plane, causing blurriness and image degradation. This effect is exacerbated when imaging 3D samples such as cells that contain liquid-filled areas that scatter light, causing information to be lost.
Confocal Microscopy
Confocal microscopy improves on standard fluorescence microscopy by using pinholes to reject out-of-focus light (figure 1), which results in greater resolution, greater contrast and reduced noise. However, this pinhole only images a tiny area of a sample (approx. 100 nm) and consequently needs to be scanned across the whole sample, which takes time and can cause photo damage. This format is known as laser scanning confocal microscopy (LSCM).

Image obtained from Leica Science Lab.
Spinning Disk Confocal Microscopy
Spinning disk confocal microscopy (SDCM) represents an alternative to LSCM. Rather than a single pinhole, a SDCM has hundreds of pinholes arranged in spirals on an opaque disk (figure 2), which rotates at high speeds. When spun, the pinholes scan across the sample in rows, building up an image. Using a spinning disk vastly improves the speed of image acquisition (allowing for imaging of fast dynamic processes and live specimens), and considerably reduces photo damage.
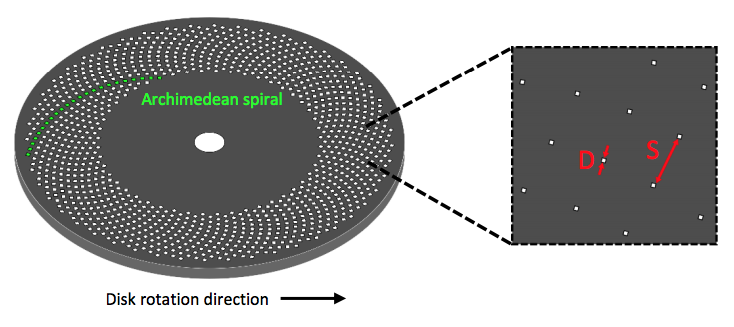
The pinholes in the disk are arranged so that every part of the image is scanned as the disk is spun. By altering the disk rotation speed, pinhole diameter and/or pinhole spacing; the image brightness, contrast and quality can be optimized, making SDCMs highly customizable. However, while larger pinhole diameters results in improved light transmittance through the disk it also reduces resolution, and while having big pinhole spacing eliminates any pinhole cross-talk, it also reduces light transmittance.
Having small pinholes with large spacing would result in higher resolution but the lowest transmittance of light through the disk. Transmittance can be improved with the addition of a second disk which contains micrometer-scale lenses in the place of pinholes. The micro-lens disk focuses light through each pinhole of the primary disk, with considerably improves light transmittance to the sample, as seen in figure 3.
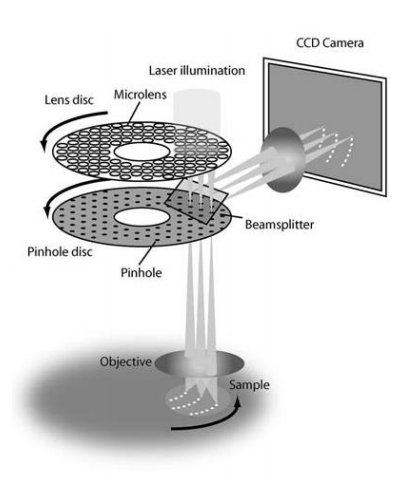
Image obtained from Graf et al. 2005.
Cameras For Spinning Disk Confocal Microscopes
An important point to consider is that SDCMs excite/image multiple points at once and can spin at very high speeds, meaning that high-quality scientific cameras are needed in order to capture images from the sample. These cameras need to have high framerates due to the speed of the spinning disk, because longer exposure times or a slow disk rotation speed may result in a streaky image. By using cameras that can match high rotation speeds, high-quality images are produced, as seen in figure 4.
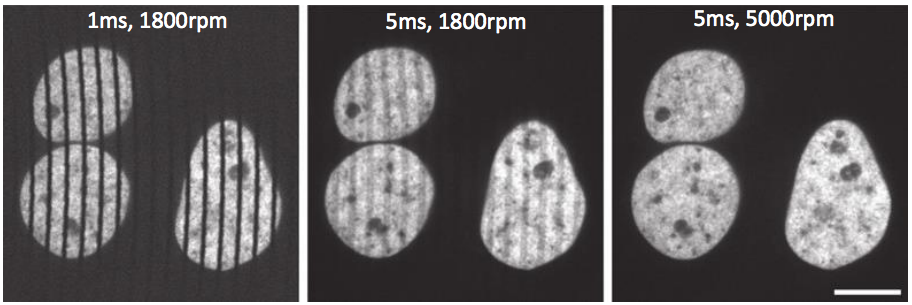
Image obtained from Stehbens et al. 2012.
Summary
SDCM offers a clear improvement on LSCM and conventional fluorescence microscopy, allowing for fast and efficient imaging of live samples, dynamic processes and optical sectioning of 3D samples in 2D slices. Multichannel images can be taken at multiple wavelengths, resulting in high-quality images dense with information, and deconvolution methods exist to further improve contrast and image quality.
The main disadvantage of SDCM is that only a small proportion of light is transmitted through the disk to the sample, but with customizable pinhole disks, secondary micro-lens disks and highly sensitive cameras these limitations can be easily overcome, while also improving imaging speed and field of view even further to produce high resolution images faster than ever.
