Dr. Simon Berger
Prof. Alex Hajnal
Department of Molecular Life Sciences, University of Zurich, Switzerland
Background
The laboratory of Prof. Alex Hajnal study the model organism Caenorhabditis elegans, the nematode worm, in order to understand biological processes such as organogenesis, the mechanisms involved, and how these relate to other areas such as cancer research. Traditionally, C. elegans imaging is done by sandwiching the worms between glass slides and using drugs/chemicals to immobilize the worms for easier imaging. However, these are not physiological conditions since only an actively moving worm can develop and function normally.
Dr. Simon Berger of the Hajnal lab has developed microfluidic devices to fine-tune the experimental environment, safely securing the worms for efficient imaging while also ensuring they can behave in a normal physiological manner, without affecting normal development. Dr. Berger works with many live worms in a single microfluidic device, imaging through the transparent material and taking 3D z-stacks of worms while they are actively moving.
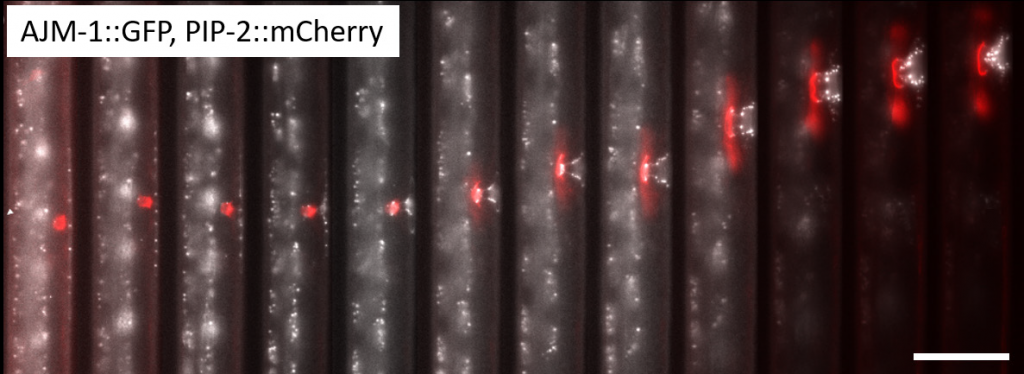
Challenge
When working with live organisms it is vital to keep the light level as low as possible in order to avoid photodamage or bleaching of the sample. However, without a sensitive camera, it can be difficult to collect enough signal while operating at a low light level.
In addition, as the worms are active and moving, it is vital to image at a high speed in order to avoid motion artifacts. High speed imaging would require a lower exposure time, meaning the signal levels are lower still, so this high speed is also dependent on a highly sensitive camera.
Having a large field of view would also allow for as many worms as possible to be captured in one field, streamlining experimentation. Having a small pixel size would allow for optimal resolution at lower magnifications, increasing both the potential field of view and the light level.
Finally, these experiments are set up in a complex way, with advanced triggering needed to control LEDs and the piezo movement in order to capture the worms as fast as possible.
“The advantage of the [Prime] BSI is you have high quantum efficiency, therefore you can image with lower illumination intensities and short exposure times for longer periods of time without damaging the animals.”
Solution
The Prime BSI back-illuminated sCMOS is the ideal camera for this application, offering high sensitivity, high speeds, and optimized pixel size.
As stated by Dr. Berger: “The advantage of the [Prime] BSI is you have high quantum efficiency, therefore you can image with lower illumination intensities and short exposure times for longer periods of time without damaging the animals.” Dr. Berger also enjoyed the high imaging speeds: “Comparing the Prime BSI to an EMCCD, you also benefit from being able to image much faster at higher framerates, which means even if the worm still moves while you acquire a z-stack it doesn’t matter as much, as you take it faster.”
The 6.5 μm pixel was also a benefit as it allowed for lower magnifications: “Nowadays with the [Prime] BSI I can image more or less anything with 40x and get higher intensities and field of view, this amplifies the benefit of higher quantum efficiency.” Previous 72% front-illuminated sCMOS cameras and EMCCDs were insufficient for this application due to the low sensitivity and low speeds respectively, overall the Prime BSI is the model camera for studying this model organism.

