Introduction
Light microscopes are used to observe and image objects that are too small for the human eye to see properly. However, there is also a limit to what these microscopes can see, as they are limited by the diffraction limit of light and can only observe/image samples that are larger than approximately 200 nm in size.
Human cells are typically 10 µm in diameter, meaning that microscopes can easily observe them but smaller components inside the cell (mitochondria, nucleus, etc.) and other organisms like bacteria and viruses can be over 100x smaller, and other samples like DNA and proteins can be smaller still. If researchers want to observe these small samples they can’t do it with normal ‘diffraction-limited’ microscope techniques, and need techniques that can break through this diffraction limit: ‘super-resolution’ techniques.
The Diffraction Limit Of Light
The smallest point that can be seen with a diffraction-limited system is called an Airy disk, named after the mathematician George Biddell Airy. Because light travels as a wave, when it passes through the small microscope aperture the light spreads out into a diffraction pattern, spreading the light out over a wide area, as seen in Fig.1.
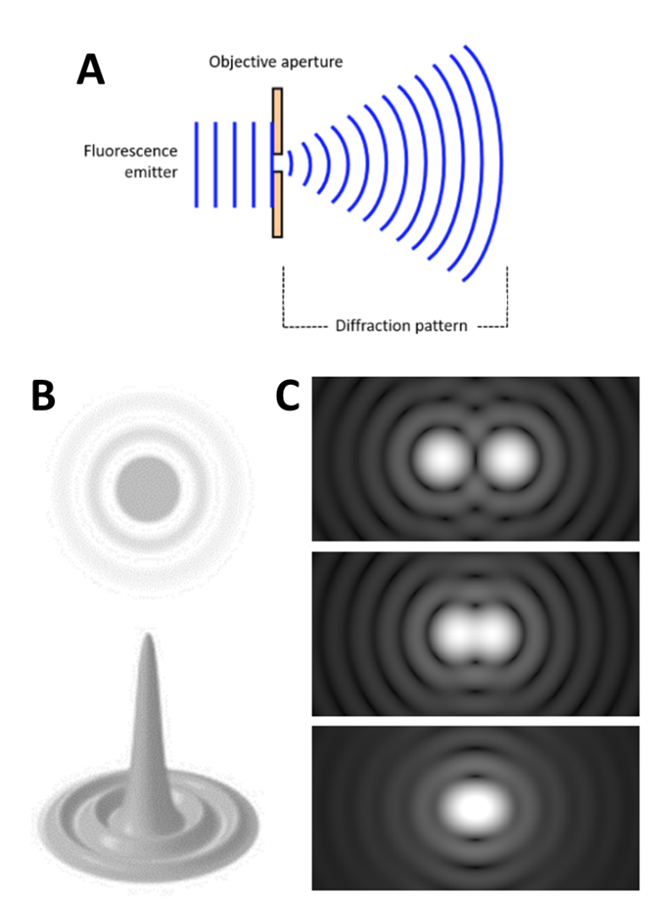
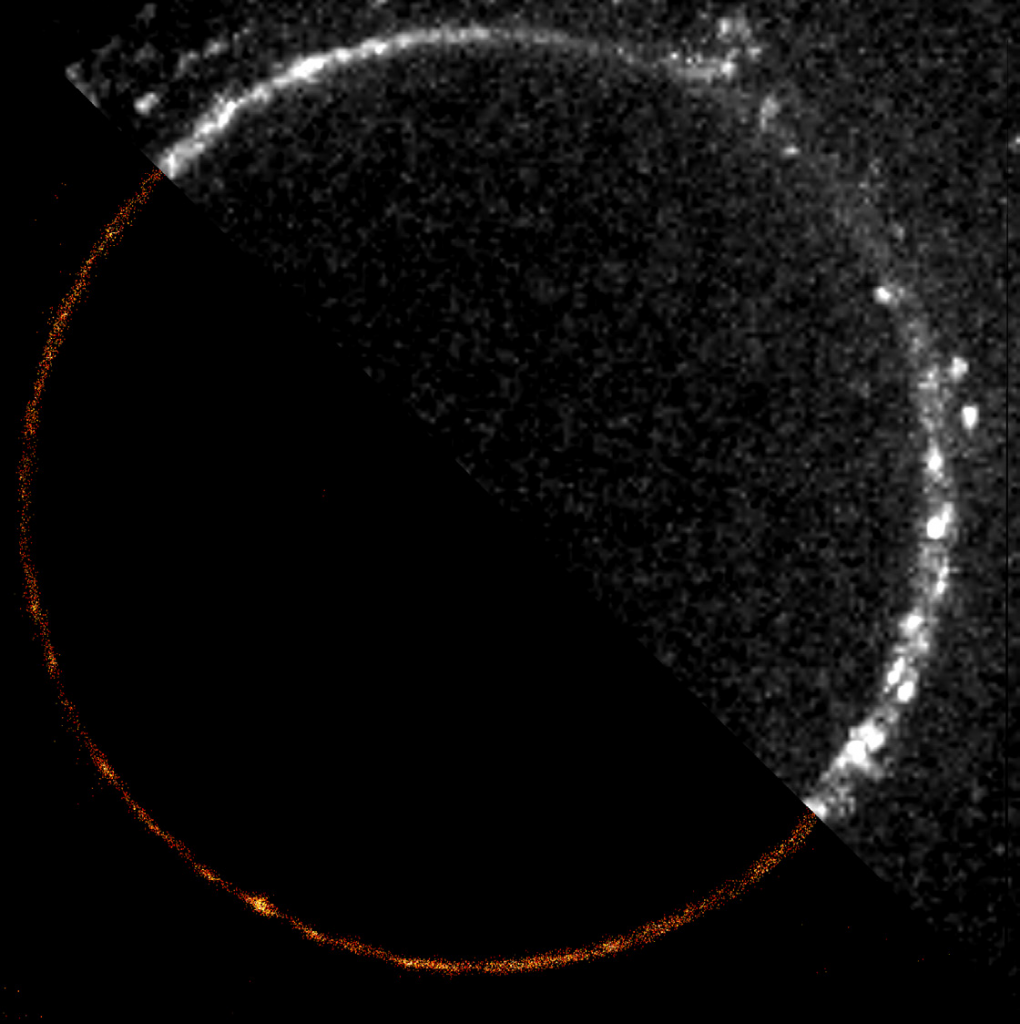
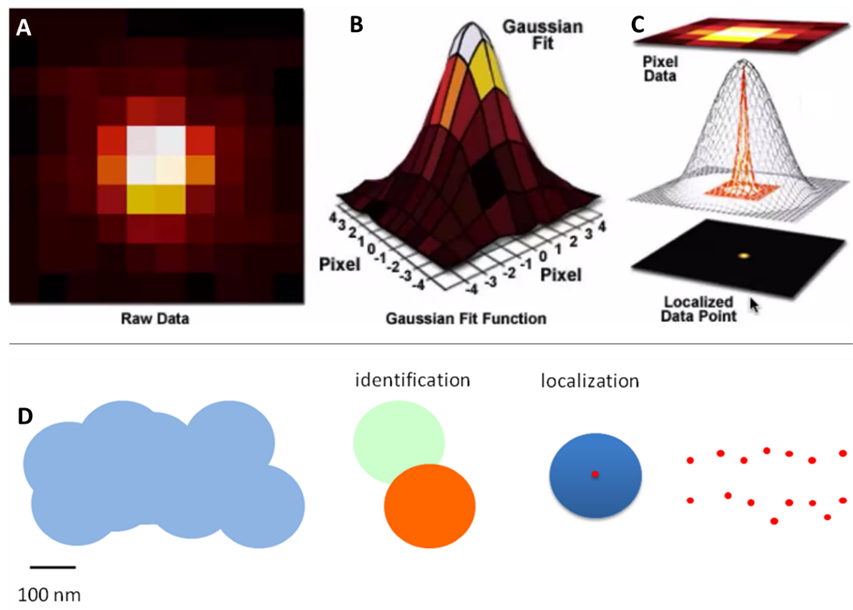
The spreading light forms an Airy disk (Fig.1B) and illuminates everything across a large area, making it impossible to pick out smaller sub 200 nm sized structures. Statistics can be used to convert the Airy disks into smaller points: because an Airy disk is brighter at the center, a point is fitted to the brightest spot. This point is accurate up to 1 nm and can improve image quality (Fig.2).
However, the main issue is highlighted in Fig.1C: if two Airy disks get too close together they can merge into one, making it impossible to tell close objects apart. These merged disks are counted as one data point and information is lost, even with the statistical fitting. Microscope samples contain millions of molecules that can be various distances apart, if they are too close they will be observed as a single point instead of multiple points. Only with super-resolution techniques can these samples be properly imaged.
Super-Resolution Techniques
Super resolution techniques use a variety of methods to break the diffraction limit of light, and can be broken down into a few main categories.
Super-Resolution Localization
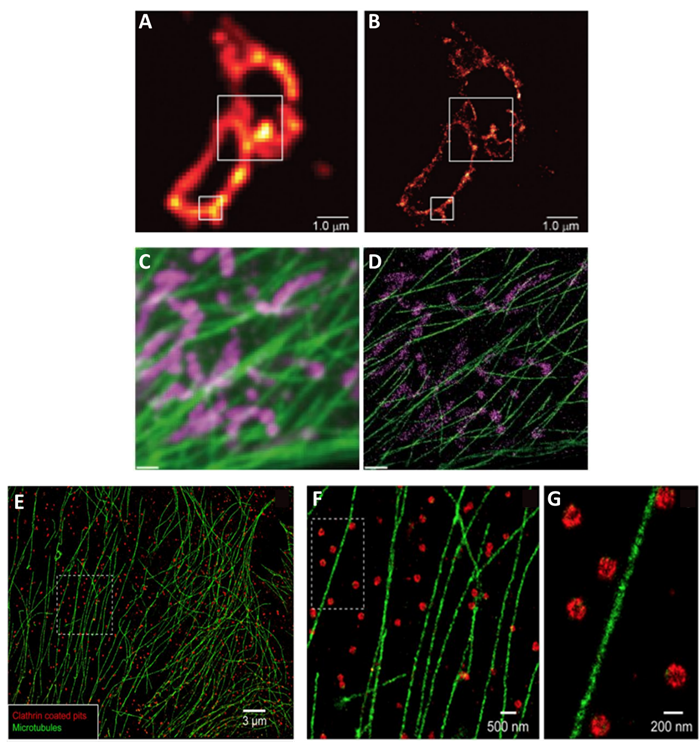
Localization techniques overcome the problem of overlapping fluorophores by using fluorescent molecules that switch on and off at random. By taking thousands of images enough data is captured where all the molecules would have been “on” at least once. Molecules close together in space, are unlikely to both be “on” at the same time in the same image frame and can therefore be separated by time. Techniques in this category include: photoactivated localization microscopy (PALM) (seen in Fig.3A-B), stochastic optical resolution microscopy (STORM) (seen in Fig.3E-G), and DNA-based point accumulation for images in nanoscale topography (DNA-PAINT) (seen in Fig.3C-D).
Structured Light
Structured light techniques use patterns of light instead of normal illumination. The basic technique in this category is structured illumination microscopy (SIM). When two patterns are overlaid it creates an interference pattern called a Moiré pattern, by imaging through these Moiré patterns super-resolution images can be achieved. A useful variation of SIM is instant SIM (iSIM) which is up to 10,000x faster than PALM/STORM and uses patterns of light that are scanned across the sample. Examples of iSIM images are shown in Fig.4. This technique is similar to spinning disk confocal microscopy, which can also achieve super-resolution imaging.

Super Resolution Spinning Disk
Spinning disk confocal microscopy is a versatile and widely used imaging technique in biology due to its ability to perform fast, 3D imaging of live cells. Recently, techniques have been created that combine super-resolution imaging with the simplicity and optical sectioning capability of spinning disk confocal, resulting in a spinning disk system capable of a twofold resolution improvement over the diffraction limit. Combining super-resolution with spinning disk confocal microscopy overcomes many issues of both techniques to create a powerful new technique for live cell imaging that promises high sensitivity, resolution, and speed.
Through a process of optical photon reassignment, this technique increases the maximum resolution of a spinning disk confocal by up to √2x (1.4x), up to 2x after deconvolution. By rotating the disks at 4000 rpm, super-resolution images can be captured live at up to 200 fps using most fluorescent dyes.
These super-resolution systems have a maximum resolution limit of ~120 nm (as opposed to the diffraction-limited SDCM maximum of ~250 nm). Due to this high resolution, it is necessary to pair these systems with a camera that is also capable of sampling at super-resolution levels. It is vital to use an appropriate scientific camera for super-resolution SDCM with the pixels optimized for Nyquist sampling at higher magnifications, as this kind of system uses very high magnifications, often over 200x by combining objectives.

Summary
By breaking through the diffraction limit of light, super-resolution microscopy can image small samples with high resolution, pushing the boundaries of scientific research.
In order to read more about each class of super-resolution technique, be sure to check the individual app notes on the Learn page:
- Localization (PALM, STORM, DNA-PAINT)
- Structured Light (SIM and iSIM)
- Super-resolution spinning disk
